The function of colorants on various pharmaceutical dosage forms usually consist of various reasons: (1) to enhance aesthetic appearance hence sometimes referred to as cosmetics for pharmaceutical preparations; (2) to impart distinct appearance for easy identification of a particular formulation thereby decreasing dispensing/medication errors; (3) to increase patient acceptability of medications especially for children; and (4) to prolong stability by providing opacity to protect light-sensitive active materials in the formulation.
The following pharmaceutical preparations are commonly imparted with colorants/coatings:
As per the Food Drug and Cosmetic Act of 1938, three categories of coal tar dyes were created, of which only the first two are applicable to the manufacture of chewable tablets.
In general, inorganic pigments and lakes possess a relatively low hazard, and depending on the complexity of the process and the quantity of materials being handled, standard chemical handling precautions should be observed. Special care must also be taken in preventing excessive dust generation and inhalation of dust. On the other hand, organic dyes, natural colors, and nature‐identical colors present a relatively greater hazard and appropriate precautions should accordingly be taken.
In addition, observance on guidelines set by FDA on the current good manufacturing practices (cGMP) applicable to drug, food and cosmetics must be practiced in order to ensure that products being processed are maintained to be of safe and high quality, and are not adulterated.
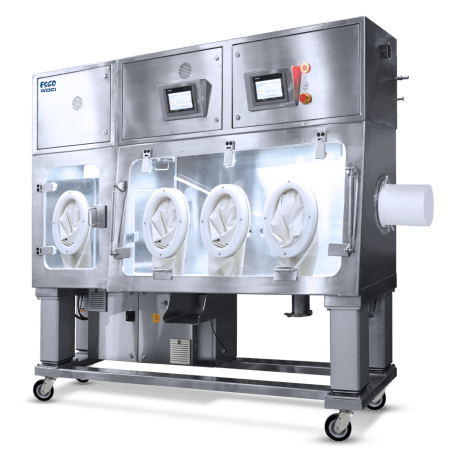
Esco offers a wide range of products that allows safe and efficient handling/ compounding of materials that may pose health hazard risks. Examples of these include Isolators and Ventilated Balance Enclosures.
Maintenance of controlled room condition and safety is enhanced with pass boxes, pass-through, and air showers at the exit, and/or in between the controlled room and its external environment.
Esco Healthcare with its innovative and turnkey solutions, backed with its four (4) core technologies, enables various industries such as pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
The following pharmaceutical preparations are commonly imparted with colorants/coatings:
- Tablets (core/ coating)
- Capsules (capsule shell/ coated beads)
- Oral liquids
- Topical creams
- Ointments
- Salves
- Organic dyes and their lakes
Dyes Lakes - synthetic, chemical compounds that exhibit their coloring power (tinctorial strength) when dissolved in a solvent.
- Dyes are soluble in either propylene glycol or glycerin.
- Their physical properties (e.g., particle size) are usually not considered critical in terms of their ability to produce identically colored systems, and their tinctorial strength is directly proportional to their pure dye content.
- Colors for clear liquid preparations are limited to the dyes. Examples of these include Tartrazine, Erythrosine, Sunset Yellow, and Patent Blue V.
- defined by the US Food and Drug Administration (USFDA), as
"Aluminum salts of food, drugs and cosmetic (FD&C) water soluble dyes extended on a substratum of alumina." - Lakes are insoluble and color by dispersion, and unlike dyes their particle size are critical to their tinctorial strength where smaller particle size impart higher tinctorial strength due to increase in surface area for reflected light.
- Advantages in using lakes include reduced mottling in tablets due to opacity of the system, thus, minimizing the defect of tablet surface depressions, and time-saving color development as it is easily achieved in less number of application states. Examples of these include Brilliant Blue Lake, Sunset yellow lake, Amaranth lake, Allura red lake, Indigo carmine lake, and Quinoline yellow lake.
- Inorganic colors or mineral colors
Inorganic colors or mineral colors offer great advantage with their opacifying capabilities (e.g., Titanium dioxide) which enhances formulation or drug stability towards light. Although there is only a limited selection of colors, these colorants are preferred due to their wide regulatory acceptance.
Significant applications of inorganic/mineral colors to pharmaceuticals include mixture of red and yellow ferric oxides to give calamine a flesh color, and use of Titanium dioxide to color and opacify hard gelatin capsules. - Natural colors or vegetable and animal colors
Natural colors or vegetable and animal colors is a chemically and physically diverse group of materials. Some colors are products of chemical synthesis (e.g., β‐carotene) rather than extraction from a natural source, and the term “natural” refers to “nature identical” and is used more descriptively. In general, although natural colors are not stable to light as compared to other groups of colors, they are advantageous due to their wide acceptability.
In application to medicaments and pharmaceuticals, only few vegetable extracts have acceptable colors of their own. In the past, large number of colorants of vegetable origin were used, but presently only three (3) are left in the codex: caramel (formerly called burnt sugar), cochineal (a dried insect), and carmine (the aluminum lake of the coloring matter of cochineal). Other examples of natural colorants include the following:
- Riboflavin and Anthocyanins
- Paprika Oleoresin
- Beet Root Red
- Curcumin (Turmeric)
As per the Food Drug and Cosmetic Act of 1938, three categories of coal tar dyes were created, of which only the first two are applicable to the manufacture of chewable tablets.
- FD&C colors: These are colorants that are certifiable for use in foods, drugs, and cosmetics.
- D&C colors: These are dyes and pigments considered safe for use in drugs and cosmetics when in contact with mucous membranes or when ingested.
- External D&C colors: These colorants are only considered safe for external use due to their oral toxicity, hence not certifiable for use in products intended for ingestion.
In general, inorganic pigments and lakes possess a relatively low hazard, and depending on the complexity of the process and the quantity of materials being handled, standard chemical handling precautions should be observed. Special care must also be taken in preventing excessive dust generation and inhalation of dust. On the other hand, organic dyes, natural colors, and nature‐identical colors present a relatively greater hazard and appropriate precautions should accordingly be taken.
In addition, observance on guidelines set by FDA on the current good manufacturing practices (cGMP) applicable to drug, food and cosmetics must be practiced in order to ensure that products being processed are maintained to be of safe and high quality, and are not adulterated.
Esco offers a wide range of products that allows safe and efficient handling/ compounding of materials that may pose health hazard risks. Examples of these include Isolators and Ventilated Balance Enclosures.
Maintenance of controlled room condition and safety is enhanced with pass boxes, pass-through, and air showers at the exit, and/or in between the controlled room and its external environment.
Esco Healthcare with its innovative and turnkey solutions, backed with its four (4) core technologies, enables various industries such as pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
References:
- Biswal, P., Mishar, M., Bhadouriya, A., & Yadav, V. (2015). An Updated Review on Colorants as the Pharmaceutical Excipients. International Journal of Pharmaceutical, Chemical and Biological Sciences, 5(4), 1004-1017. Retrieved from: https://www.researchgate.net/publication/322861568_An_updated_review_on_colorants_as_the_pharmaceutical_excipients
Recommended Products