Formulation and Filling Line
Esco partners with filling line companies to create a client-specific technology, which utilizes cGMP compliant isolators and high quality filling line accessories/technologies, to ensure product safety and sterility throughout the entire manufacturing cycle.
Enclosure systems for this technology can range from open and closed Restricted Access Barrier Systems (o/cRABS) to leak tight isolation technologies compliant to international GMP standards.
Flexible Multi-format Ready-to-Use (RTU) Filling Line System
Integrates the best industry technologies to simplify the manufacturing process. It utilizes flexible ready-to-use and single-use consumables, thus, removing container preparation process at the manufacturing site. It can also cater to multi-format containers and sizes on a single modular platform.
Combined manufacturing process that allows multiple container formats to be filled and finished on a single system.
The use of RTU and single-use components lessens process delays and run time, as well as increases sterility of the process.
-
Subtypes:
-
Single Format
Robotic
Non-Robotic
-
Multi-Format
Robotic
Non-Robotic
-
Flexible Multi-format Ready-to-Use (RTU) Filling Line System Integrations:
RTU consumables
Single-Use Disposable Components:
Mixing bag/Holding bag
Pre-filtration sampling bags
Reservoir bags
Aseptic connections/assemblies
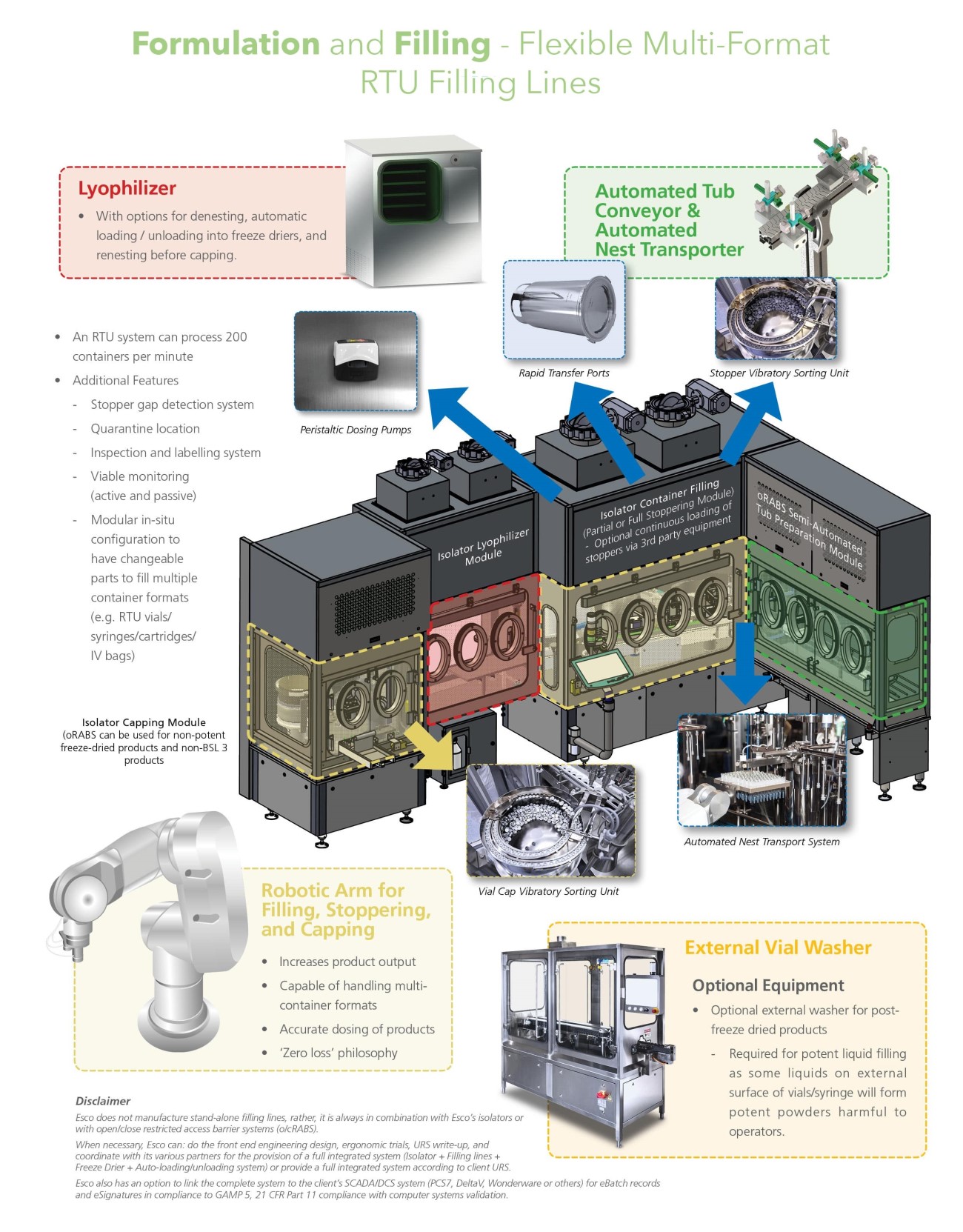
Automated Tub Conveyor & Automated Nest Transporter
Robotic Arm (filling, stoppering, and capping)
External Vial Washer
Additional Features
Stopper gap detection system
Quarantine location
Inspection and labelling system
Viable monitoring (active and passive)
Modular in-situ configuration to have changeable parts to fill multiple container formats (e.g. RTU vials/ syringes/cartridges/IV bags)
-
Flexible Single-Format RTU Filling Line System: Non-Robotic
Incapable to have an in situ format change
(i.e.: vial to syringe or to cartridge, vice-versa).Optional external washer for post-freeze dried products
Required for potent liquid filling as some liquids on external surface of vials/syringe will form potent powders harmful to operators.Highly efficient for high volume dedicated products where flexibility is not necessary
-
Flexible Single-Format RTU Filling Line System: Robotic
-
The use of robotic systems increases accuracy and repeatability of the process which is a high demand for aseptic processes
-
Advatages:
Full enclosure within an isolator system
Ready-to-use containers
Robotic Arms
-
Features:
Optional external washer for post-freeze dried products
Required for potent liquid filling as some liquids on external surface of vials/syringe will form potent powders harmful to operators.Incapable for an in situ format change
(i.e.: vial to syringe or to cartridge, vice-versa).
-
-
-
Flexible Multi-Format RTU Filling Line System: Non-Robotic
-
Ability to fill multiple container formats & sizes on a single modular platform
-
Increased asset utilization
-
-
Flexible Multi-Format RTU Filling Line System: Robotic
-
Utilizes the best automation technologies in the industry to increase accuracy and throughput of multiple container systems.
-
Advatages:
Flexible - compatible with new or changing products
Less complex
Smaller more flexible facility
Reduced intervention risk
Isolator-barrier technology
-
Features:
Isolator Barrier Technology + Robotics
Provides full separation between the operator and the process
Compatible with VHP bio-decontamination
Recipe driven operation
Maximum flexibility and functionality
Negligible particle generation
-
-








