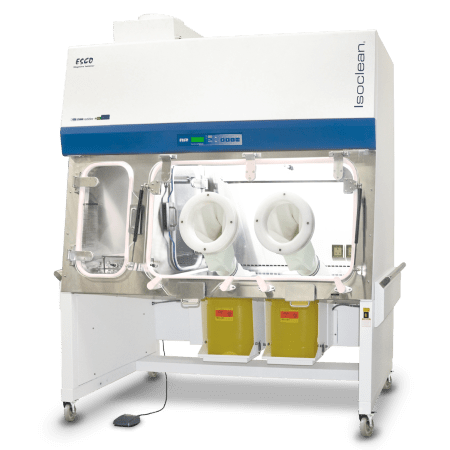
Designed in accordance with the United States Pharmacopeia General Chapter <797> guidelines, Esco Healthcare’s CAI ensures a sterile/aseptic compounding environment within the isolator at all times, from compounding up to material transfer operations.
Understanding CAI
Esco Healthcare’s Compounding Aseptic Isolator (CAI) provides a safe and clean environment for compounding of non-hazardous, sterile drug preparations and IV admixtures in compliance with USP <797> criteria.
The work zone and pass-through interchange are under positive pressure to the room to maintain sterility in case of breach in the barrier isolation system. Air cleanliness is maintained with the use HEPA filters and other filters capable of eliminating microbial and other particulate contaminants
Key Applications:
Aseptic Compounding
Total Parenteral Nutrition Formulation
Sterile Filling Line
Cell Therapy
Gene Therapy
Tissue Engineering
Batch Sterility Testing
Cosmetic / Cosmeceuticals
Advantages of Esco Healthcare’s CACI
One of the requirements of USP <797> is to ensure that air from the ambient environment going into the isolator must first pass through microbially retentive filters (HEPA at minimum). As such, Esco Healthcare’s Compounding Aseptic Isolators (CAI) are designed with a standard HEPA (H14) filtration system with a 99.995% filtration efficiency at 0.1 to 0.3 microns, thus, providing an ISO Class 5 air quality in the chambers (as per ISO 14644-1).
Pressure Regime
For CAI and sterile compounding, the system operates under a standard positive pressure of + ≥37 Pa in the work zone and + ≥25 Pa in the pass-through chamber. This design guarantees the removal of cross-contamination risk from the surrounding environment towards the isolator chambers, which in turn ensures the provision of sterile and high-quality final compounded sterile products (CSPs).
Airflow Regime
Recirculating airflow can be applied for compounding non-hazardous sterile drugs. In a recirculating airflow regime, about 90% of HEPA-filtered air is recirculated within the isolator while approximately 10% of air is exhausted through such filters to prevent heat build-up in the system. The exhausted air will then be replenished by ambient air coming from the top in-let G4 pre-filters with 80% efficiency.
Ensuring Sterility
Sterility is key in compounding aseptic/sterile products as any contamination to the final product may result to a serious adverse event for patients which may even cause irreparable damage to the image of the institution.
CAI systems help ensure maintenance of sterility as they provide complete separation between the operators/surrounding environment and the actual process. As such, unlike open-front cabinets (laminar flow benches and biological safety cabinets), CAI units are not highly dependent on aseptic technique of the operator, although proper training and annual retraining of all operators involved in sterile compounding, must still be observed.
Another way of ensuring sterility in the work zone of the CAI is to have a proper maintenance service of the isolator system. Guidelines suggest annual recertification of the unit is important to ensure that it still provides the same quality of service similar to its integrity during initial installation.
In line with this is the importance of having a proper cleaning/decontamination process for the isolator system. Note that the frequency of this will depend on each facility’s services.
Decontamination Process
involves the proper removal of residues in the work zone through the use of agents such as: sterile alcohol, sterile water, peroxide, or sodium hypochlorite.
Cleaning Process
is the step of removing both inorganic and organic materials from the work zone via germicidal detergents and sterile water.
Disinfection Process
this is the act of destroying microorganisms that might be present in the work zone through the use of sterile alcohol or other EPA-registered disinfectants.
Each compounding facility has its own protocols for various processes, and understanding the needs of each process is critical in choosing the correct equipment that will help protect the actual sterile compounded product as well as the operator and the environment from untoward exposure or cross-contamination.
References:
- USP Compounding Expert Committee, (nd). General Chapter <797> Pharmaceutical Compounding – Sterile Preparations. Retrieved from: https://www.usp.org/compounding/general-chapter-797 [Accessed 11 November 2020].
- USP Compounding Expert Committee, (nd). General Chapter <800> Hazardous Drugs – Handling on Healthcare Settings. [ONLINE] available at: http://www.usp.org/sites/default/files/usp/document/our-work/healthcare-quality-safety/general-chapter-800.pdf. [Accessed 11 November 2020].
Related Products