

Discovery to Delivery
› Solutions ›By Drug Development Process
In accordance with the federal Food, Drug and Cosmetics Act, as regulated through Title 21 of the U.S. Code of regulations, a new drug is only allowed to be legally released to the interstate commerce once it has been approved by the Food and Drug Administration (FDA).
New chemical entities (usually small-molecule drugs) and new biological entities follow similar developmental schemes. The process and time course for the development of new drug, from the time of its discovery to its approval and marketing, can be seen as shown in the figure below and can be broken down into the following steps:
Discovery → Preclinical Studies → Investigational New Drug Application (IND) → Clinical Trials → New Drug Application (NDA) → Postmarketing
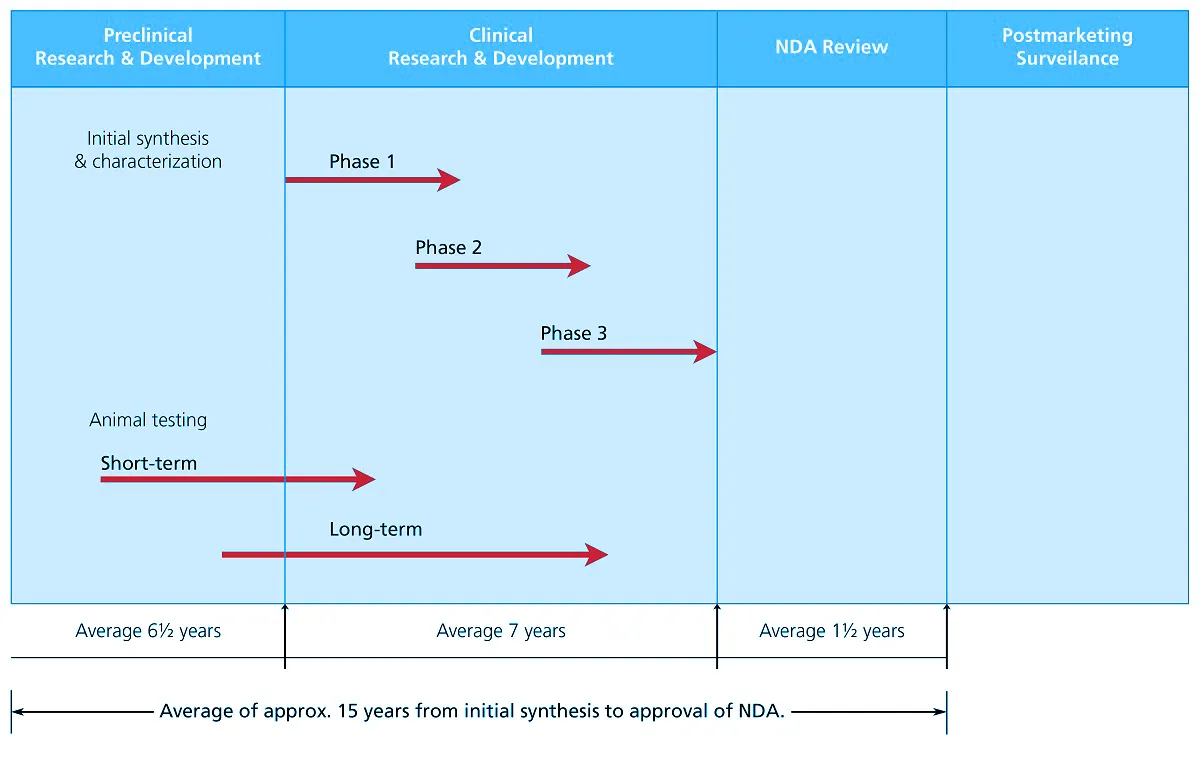
Figure 1. Time course for new drug development. From Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (pg. 36), by Loyd Allen & Howard Ansel, 2014 Lippincott Williams & Wilkins.
In order to gain an FDA approval, safety and efficacy of the new drug or drug product must be proven through use of supporting scientific evidences. In addition to this, demonstration of proper control and validation of processes and controls undertaken must be done to ensure that the product meets the established standards of quality.
With aims to maintain the quality standards of the drug product while ensuring safeguard protection of the working personnel and the surrounding environment, Esco Healthcare is backed with its 4 core technologies (Isolation Containment, Airflow Containment, Ventilation Containment and Cross-contamination Facility Integrated Barrier) allowing it to cater to even the most critical manufacturing and industrial processes.
Reference
Allen, L., & Ansel, H. (2014). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (10th ed.). Lippincott Williams and Wilkins.
Recommended Products

A New Molecular Entity (NME) is defined by the U.S. Food and Drug Administration (FDA) as an active ingredient that has never been marketed before in any form. However, drugs need not to be a new chemical entity for it to be considered new, as changes in an old and established drug may be considered novel if modifications applied to it introduce a question to its safety or efficacy, such as in the following circumstances:
Alteration in an approved drug product’s formulation or manufacturing method
Combination of two or more old drugs or alteration in a usual combination product
Proposition of new indication, new dosage schedule or regimen, new route of administration, and/ or new dosage form for an established drug
Throughout history, some drugs were found as a result of fortuitous discovery, but most as a result in the tireless pursuit of carefully designed research programs of screening, molecular modification and mechanism-based drug design. The discovery of a new chemical entity involves processes such as (1) isolation from plants, (2) synthesis in the laboratory or organic synthesis, and (3) creation through the process of biotechnology or molecular modification. Nowadays, new drugs are being discovered as a result of a more targeted chemical or engineered biologic material research; which is, directed specifically toward the identified physiologic/ metabolic process or biomolecular target of a disease.
Out of 270,000 known plants, only a few have been investigated for its medicinal activity. Plants have long serve as a reservoir, and are one of the main sources of potential new drugs. Once the active constituents have been isolated and structurally identified from the plant, organic chemists may either recreate them through total laboratory synthesis or, more importantly, use the natural chemical as a starting material in the creation of a slightly different molecularly-modified chemical. Depending on the nature and extent of the chemical alteration, these new structures which are called semisynthetic drugs, may have a slightly or vastly different pharmacologic activity from that of the starting substance. Other plant constituents that are inertly inactive or rather unimportant therapeutically may be chemically modified to yield important drugs with profound pharmacologic activity.
On the other hand, animals have served humans in their search for drugs in numerous ways. Not only have they yielded to drug testing and biologic assay, but have also provided drugs that are mannered from their tissues or through their biologic processes. More than this, use of animals also holds lifesaving significance in the production of various biologic products (e.g., serums, antitoxin, and vaccines). New vaccines for diseases such as AIDs and cancer have been developed through the use of cell and tissue cultures.
In the new era today, the pharmaceutical product development is seen as a result of the advent of genetic engineering, the submicroscopic manipulation of the double helix, the spiral DNA chain of life. Through this process, more abundant and vastly purer antibiotics, vaccines and yet unknown chemical and biologic products to combat human diseases have yet to come.
Another new promising technology is the human gene therapy which entails the transfer of a new genetic material to the cells of a patient with a genetic disease. It is used to prevent, treat, cure, diagnose, or mitigate human diseases caused by genetic disorders. Gene therapy is a medical intervention that is based on the modification of the genetic material of living cells. Cells may be modified outside the body (ex vivo) for subsequent administration, or they may be modified within the body (in vivo) by gene therapy products given directly to the patient.
In dealing with chemicals whose properties have yet to be studied, proper engineering controls must be utilized in research laboratories to ensure safety throughout the whole developmental process.
Esco Healthcare provides specialist services, equipment packages, and process solutions from our core platform products. This leads to improved protection of operators, reduction of cross-contamination, and a more efficient processing; thereby, directly and indirectly advancing occupational health and human healthcare.
Reference
Allen, L., & Ansel, H. (2014). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (10th ed.). Lippincott Williams and Wilkins.
Recommended Products

Once a new chemical entity has been discovered, it must undergo pre-clinical testing to determine whether it possesses adequate features of safety, and sufficient promise of usefulness to be pursued as a prospective pharmaceutical drug product. The proposed new drug is then biologically characterized to determine its pharmacologic and toxicologic effects.
Pre-clinical testing involves the effort of various scientists and takes about six and a half (6.5) years to be completed. It deals with the initial synthesis and characterization of new drug which includes the study on the following:
new chemical entity’s chemistry/ physical properties/ biology (pharmacology, ADME, toxicology)
preformulation
long-term animal toxicity/ animal testing
product formulation
manufacturing and controls; and,
package and label design
Preformulation studies are then conducted to define the physicochemical properties of the agent, while formulation studies follow in order to develop the initial features of the proposed pharmaceutical product or dosage form.
To acquire proof to demonstrate the drug’s safety and effectiveness for its proposed use, pharmacokinetic and pharmacodynamics studies must be completed. Scientists have developed studies that may be conducted outside the living body (ex vivo) through cell and tissue culture, and computer programs which simulate the human and animal systems. Cell cultures are being used increasingly to screen for toxicity before progressing to whole-animal testing.
When the pre-clinical studies demonstrate adequate safety and the new agent shows promise as a useful drug, the drug’s sponsor (e.g., pharmaceutical company) can file an Investigational New Drug (IND) Application with the Food and Drug Administration (FDA) for initial testing in humans (often called as phase 1 of clinical trial).
In dealing with chemicals whose properties have yet to be studied, proper engineering controls must be utilized in research laboratories to ensure safety throughout the whole developmental process.
For pharmaceutical and lab animal research, air showers can be integrated to maintain the cleanliness of the pharmaceutical production and laboratory animal breeding areas. It can also minimize egress of hazardous substances and allergens from the controlled environment.
Esco Healthcare provides specialist services, equipment packages, and process solutions from our core platform products. This leads to improved protection of operators, reduction of cross-contamination, and a more efficient processing; thereby, directly and indirectly advancing occupational health and human healthcare.
Reference
Allen, L., & Ansel, H. (2014). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (10th ed.). Lippincott Williams and Wilkins.
Recommended Products

Before proceeding on to clinical trials, application for Investigational New Drug (IND) may be submitted to the Food and Drug Administration (FDA) for one or more phases of a clinical investigation. Although the clinical trials which consist of 3 phases are conducted sequentially; certain studies may overlap.
Clinical trials last for an average of seven (7) years. Once the initial testing in humans, termed phase 1, yields enough data to guarantee adequate safety, the drug is then allowed to proceed on progressive human trials in phases 2 and 3 to further assess its safety and efficacy.
As these trials progress, laboratory work is continuously done to define the agent’s basic and clinical pharmacology, toxicology, product design and development, manufacturing scale-up and process-controls, analytical methods and development, proposed labeling and package design, and initial plans for marketing.
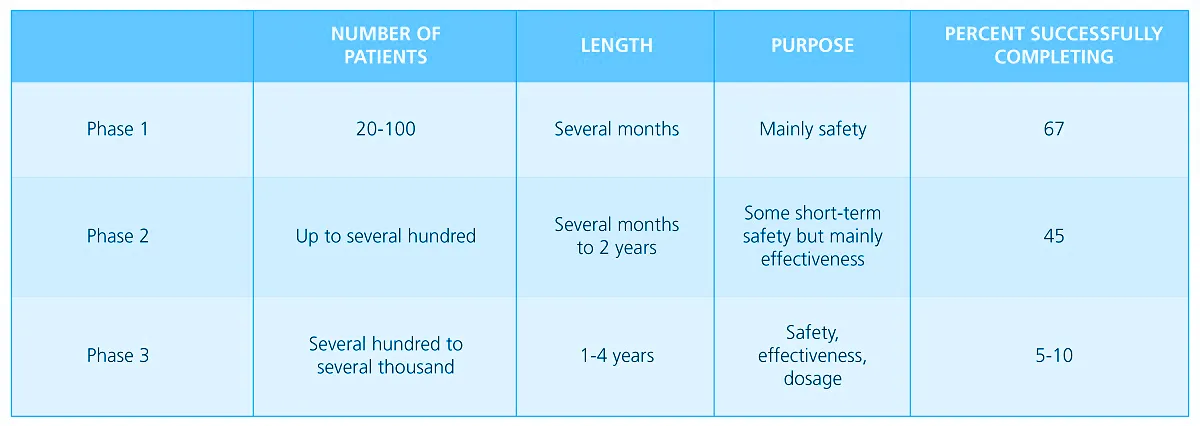
Table 1. Phases of Clinical Testing. From Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (pg. 57), by Loyd Allen & Howard Ansel, 2014 Lippincott Williams & Wilkins.
Human subjects who volunteer to participate in Phase 1 studies are usually in healthy conditions; although, there might be some exceptions due to certain protocols which necessitate patients to be their subjects. These trials typically involve 20-100 subjects who are closely monitored by clinical experts in such investigations.
Phase 1 trials deal with the initial introduction of an investigational drug into humans, and are primarily designed for the purpose of assessing its safety. Studies in this phase include the determination of the following:
human pharmacology of the drug
structure-activity relationships
side effects associated with increasing doses; and if possible,
early evidence of its effectiveness
Phase 1 studies are often used for the selection among different chemical analogs of a lead compound. Lead compound is a prototype compound which possesses the fundamentally desired biologic or pharmacologic activity of a chemical.
Once studies demonstrate sufficient merit and if the order of the drug toxicity is low, Phase 2 begins.
Unlike in Phase 1 trials which involve around 20-100 healthy volunteers, Phase 2 trials study up to several hundred patients, and since this phase has patients as subjects, side effects or toxicity symptoms that did not appear in the preclinical animal or Phase 1 studies, may be revealed for the first time.
Controlled clinical studies are conducted during this phase in order to evaluate the effectiveness of a drug in patients with the condition in which the drug is intended for and also to be able to assess the side effects and risks that may arise. Only clinicians expert in the disease being treated are used as investigators for phase 2.
Once the clinical results of Phase 2 demonstrate continued promise for the new drug with a good margin of safety then, the drug’s sponsor (e.g. pharmaceutical company) and the FDA’s review division can hold meetings for the analysis of data acquired from phases 1 and 2. Furthermore, these meetings allow resolution for questions and issues and aid in the establishment of investigational plans for Phase 3 studies.
In order to determine the drug’s usefulness in an expanded patient base, Phase 3 studies include controlled and uncontrolled trials that may be participated by several hundred to several thousand patients. Many additional clinicians with patients who have the condition for the drug’s intended use are recruited to participate in this trial.
The end goals of Phase 3 studies are to gather adequate information on the drug’s efficacy and safety for the evaluation of its overall benefit-risk relationship, and to be able to file a complete New Drug Application (NDA).
Getting an NDA approval from Food and Drug Administration (FDA) indicates that the body of scientific evidence submitted sufficiently demonstrates that the drug product is safe and effective for its proposed clinical indications, that there is adequate assurance of its proper manufacture and control, and that the final labeling accurately presents the necessary information for its proper use.
At the end of the clinical research, an NDA is filed in order to seek for approval to market the new product. This takes an average of one and a half (1.5) year to process, and is comprised of the following steps:
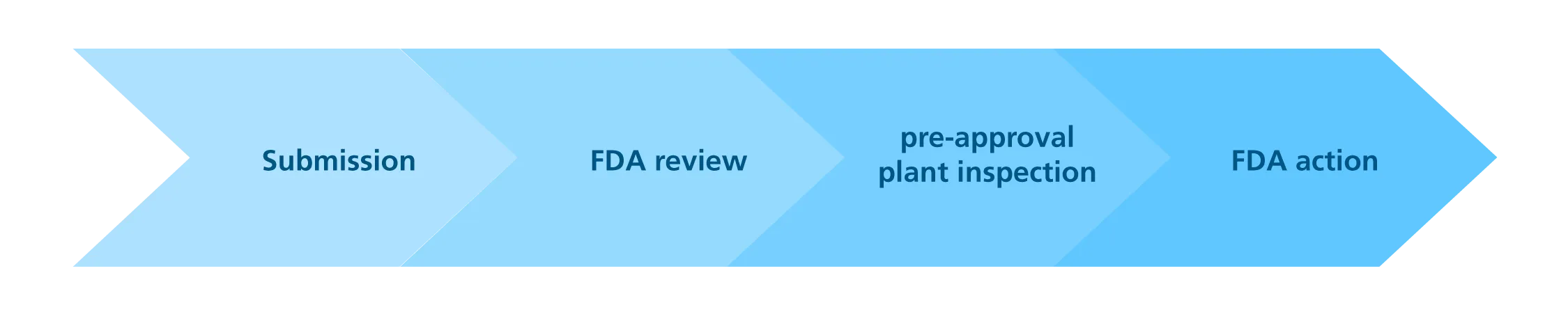
The preapproval inspection is conducted in facilities where the approved product is going to be produced. The inspectors assess the drug sponsor’s (e.g., pharmaceutical company) capability to comply with all control and quality standards contained in the application, including the FDA’s Current Good Manufacturing standards (cGMP).
Esco Healthcare with its wide range of innovative and turnkey solutions enables various industries such as pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
Reference
Allen, L., & Ansel, H. (2014). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (10th ed.). Lippincott Williams and Wilkins.
Recommended Products

Postmarketing stage collectively deals with phase 4 clinical studies, adverse reaction reporting, product defect reporting, surveys/ sampling testing, inspections, and product line extension.
After the drug’s sponsor (e.g., pharmaceutical company) receives the marketing status for a new drug product, postmarketing surveillance must continuously be ran to gather information on adverse drug reaction and product defects.
Continued clinical investigations or Phase 4 studies are conducted to learn more about the drug’s clinical pharmacology/ toxicology and additional indications. This phase may contribute to the following:
understanding of the drug’s mechanism or scope of action
indicate possible new therapeutic uses for the drug
demonstrate the need for additional dosage strengths, dosage forms, or routes of administration; and,
reveal additional side effects, serious and unexpected adverse effects, and/ or drug interactions
Serving as an adjunct to the continuing clinical studies and testing, Esco Healthcare provides specialist services, equipment packages, and process solutions from our core platform products. This leads to improved protection of operators, reduction of cross-contamination; thereby, directly and indirectly advancing occupational health and human healthcare.
Reference
Allen, L., & Ansel, H. (2014). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems (10th ed.). Lippincott Williams and Wilkins.
Recommended Products
Sign up to our newsletter and receive the latest news and updates about our products!
