BioPass™ Pass Through
BioPass™ Pass Through is floor standing airtight transfer chamber with onboard ventilation and integrated atomized or vaporized hydrogen peroxide (AHP/VHP, H2O2) based bio-decontamination system designed for passing large equipment into a ISO Class 5 cleanroom in an aseptic manner.
BioPass™ Pass Through provides a flush threshold enclosure to allow materials to be wheeled into the enclosure with minimum effort. Full stainless steel assembly in compliance with cGMP's design requirements.
Industries Served:
- Hospital
- Food, Beverages & Confectionary
- Manufacturing Facilities
- Veterinary Surgeries
- Dentist
- Primary Healthcare Facilities
- Pharmaceutical Industry
Easy-to-clean construction
The interior and cleanroom side face of the pass through cabinet/ chamber is made of 316 L stainless steel with a smooth interior and coved corners to ensure easy cleaning and biodecontamination
- The interior surface is polished to 0.8 Ra μm or better and external surfaces exposed to cleanrooms 1.2 Ra μm or better
- The cleanroom wall interface allows a flush finish with the surface for cleanliness
Interlocked inflatable seal doors
Pass through chamber doors are constructed with silicone inflatable seal. This removes the need for external mechanical latch and ensures maximal airtight containment, and high leakage resistance.
- Doors shall give > 90° opening for full access
- Interlocking doors preventing cross-contamination by not allowing sterile unloading doors from opening until after biodecontamination
Chamber pressure monitoring
Direct reading pressure gauges are provided to both sides of the pass through cabinet to give indication of the chamber pressure
H2O2 biodecontamination system
Integrated with Esco BioVap™ biodecontamination system with:
- Touch screen HMI/PLC: Enables intuitive operation and cycle monitoring with password protection and data logging
- Selection from ticket roll printer or PDF file via HMI for biodecontamination cycle
Integrated Biodecontamination System
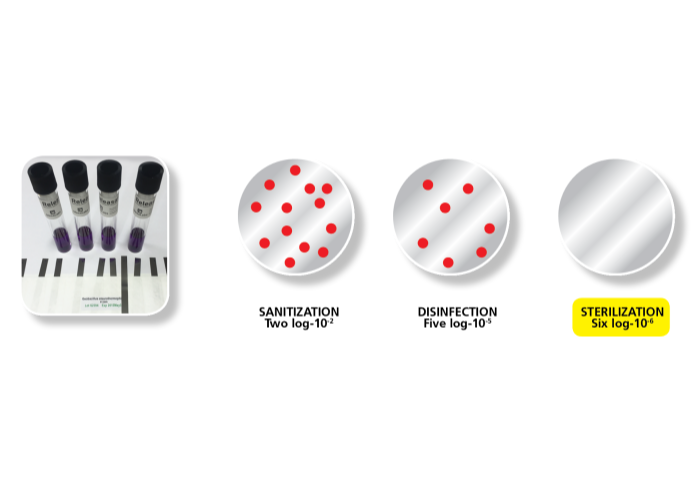
Esco Pharma has developed an effective hydrogen peroxide based Biodecontamination system capable of achieving a log 6 reduction in bio-burden. The spore log reduction has been validated by biological indicator challenge using biological indicator stainless steel ribbons populated with Geobacillus stearothermophilus spores.

|
GENERAL SPECIFICATIONS (AHP Cycle) |
|
|
Air Injection Pressure |
4 bar ± 10% |
|
Air Injection Flow Rate |
32 lpm ± 10% |
|
Injection Time |
30 sec – 20 mins |
|
Dwell Time |
15 – 45 mins |
|
Aeration Time |
20 – 90 mins
|
|
Total Decontamination |
30 mins – 2 hrs
|
|
Sterilant Used in One Cycle |
10 – 150 mL |
|
Sterilant Injection Flow Rate |
200 – 1000 µL/sec |
|
Sterilant |
30% Hydrogen Peroxide |
Safe exhaust of air
Optional on-board catalytic converter to allow air to be taken from the room, then exhausting it back, with interlocked safety exhaust H2O2 sensor. This avoids costly HVAC ducting.
Airflow Pattern
- During operation, room air is drawn through the top of the unit via HEPA H14 knife edge gel sealed filter with ≥ 99.995% efficiency in the primary side resulting in a unidirectional stream of clean air projected vertically over the internal work zone. All airborne contaminants are flushed and diluted, resulting in a particulate-free mobile work environment.
- During bio-decontamination process, the air will run through via HEPA H14 knife edge gel sealed filter with single-pass flow to remove hydrogen peroxide concentration inside the chamber during aeration stage.
- A nominal filter face velocity (client scope) of 0.45 m/s (90 fpm) ensures that there are a sufficient number of air changes within the enclosed area of the laminar flow unit in order to maintain cleanliness.
- HMI system monitors the airflow and other critical parameters such as chamber pressure, H2O2 concentration, temperature and relative humidity and provides alarms related to the critical parameters that out of the tolerance limit.
Clean Air, Purging and Biodecontamination Capabilities
BioPass™ Pass Through cabinet provides a flush threshold enclosure to allow large materials to be wheeled into the enclosure with minimum effort.
The pass through chamber’s onboard ventilation system ensures all materials passing through are purged off of contaminants, and together with its biodecontamination system, aseptic process in an ISO Class 5 cleanroom is guaranteed.
|
STANDARD INTERNAL DIMENSIONS |
|||||
|
W x D x H (mm) |
700 x 700 x 700 mm |
800 x 800 x 800 mm |
900 x 900 x 900 mm |
1000 x 1000 x 1000 mm |
1200 x 1200 x 1200 mm |
|
W x D x H (in) |
27.6” x 27.6” x 27.6” |
31.5” x 31.5” x 31.5” |
35.5”x 35.5” x 35.5” |
39.4” x 39.4” x 39.4” |
47.3” x 47.3” x 47.3” |
|
W x D x H (ft) |
2.30’ x 2.30’ x 2.30' |
2.63' x 2.63' x 2.63' |
2.96' x 2.96' x 2.96' |
3.3' x 3.3' x 3.3' |
3.94' x 3.94' x 3.94' |
|
GENERAL SPECIFICATIONS |
||
|
Air Classification |
ISO Class 5 as per ISO-14644-1 (Grade A as per EU GMP) |
|
|
Aiflow Pattern |
During normal operation |
Single Pass |
|
During biodecontamination |
·
Recirculatory (During gassing and dwelling) ·
Single Pass (During aeration) |
|
|
Operating Pressure |
+50Pa with respect to ambient environment |
|
|
Leak Tightness |
The acceptable leakage rate of the chamber will be no greater than 10-2/h,
Refer to ISO 10648-2, Class 3 Containment Enclosure. |
|
|
Lighting |
≥600 |
|
|
Noise Level |
≤75 dBA |
|
|
Temperature |
Monitored (not controlled) |
|
|
Humidity |
Monitored (not controlled) |
|
|
Filtration Elements |
Pre-Filter |
G4 |
|
Supply Filter |
HEPA (H14) filter with typical efficiency of ≥ 99.995% at MPPS |
|
|
Exhaust Filter |
||
|
Biodecontamination |
BioVap™ (Hydrogen
peroxide-based) capable of achieving a minimum of log 6 reduction in spore
forming micro-organisms validated using a BI challenge validation |
|
H2O2 Monitoring System - (One per Biopass Needed)
To ensure the concentration of hydrogen peroxide inside the chamber to confirm end of aeration.
- AHP Systems: Install a low-level sensor
- VHP Systems: Use both low-level
Room H2O2 sensor
Remote Catalytic Converter
Allows aeration of the system and operation without the need for site ducting. The system can be exhausted to the room following aeration.

Remote Catalytic Converter
How does BioPass™ Pass Through differ from other transfer chambers in the market?
With greater than 40 years of experience in the industry, Esco simply provides more comprehensive features that reflect in its withstanding core technologies and innovations that have been tested through time.
BioPass™ Pass Through, one of Esco’s developments under the Cross-Contamination Facility Integrated Barrier product line, caters to large equipment/material transfer needs in order to lessen personnel traffic while ensuring integrity of controlled environments during the transfer process.
One of its main advantages is its all-in-one comprehensive design, made with stainless steel for cGMP compliance, which includes the following:
- Onboard ventilation with HEPA filtered air
- Integrated H2O2 based BioVap™ biodecontamination system
- Catalytic converter for safe exhaust of air out of the chamber to avoid costly HVAC ducting on site
- HMI controller for 21 CFR Part 11 Compliance
How does BioPass™ Pass Through differ from other pass through cabinets offered by Esco?
Esco Pharma offers 4 types of pass through cabinets/ hatches with distinct qualities which differentiate it from one another, namely:
- Static pass boxes
These pass through cabinets and hatches are utilized and installed for aseptic transfer of materials into and out of cleanroom areas.
- Dynamic pass boxes and Dynamic Floor Laminar Hatches (DPB/DFLH)
DPB/DFLH, unlike static ones, are aseptic architectural systems that are maintained with an ISO Class 5 as per ISO 14644-1 (Grade A as per EU GMP) clean air supply within its internal chambers. This is made possible by built-in prefilter, HEPA filter and blower within the DPB/DFLH unit.
Difference between DPB/DFLH is that DPB is wall-mounted and is either provided with/ without a floor stand while DFLH is floor mounted.
- Esco air shower pass boxes (EAS-PB)
EAS-PB is similar to DPB/DFLH such that air is provided to the unit but air is supplied only for a limited duration.
EAS-PB is constructed with built in air showers and is provided with an array of stainless nozzles and high velocity air jets running at 18-30 m/s. Robustly-designed to scrub off potential surface particulate contaminants from materials being transferred between controlled environments.
- BioPass™ Pass Through
BioPassTM Pass Through is similar to dynamic floor laminar hatches such that it is floor mounted and is maintained with an ISO Class 5 as per ISO 14644-1 (Grade A as per EU GMP) clean air supply within.
Distinctly, it is equipped with an integrated biodecontamination system, and is designed to relatively wheel-in large equipment/ materials into the enclosure.
- Static pass boxes
How is BioPass Pass Through cGMP compliant?
cGMP requires surfaces used to be easily cleanable. BioPass™ Pass Through utilizes stainless steel 316L which is easy to clean, non-particulate generating, resistant to API, and less prone to corrosion.

















