

Discovery to Delivery
› Solutions ›By Drug Preparations
According to the United States Pharmacopoeia (USP), compounded sterile preparation (CSP) is a preparation intended to be sterile that is created by combining, admixing, diluting, pooling, reconstituting, repackaging, or otherwise altering a drug product or bulk drug substance. Sterility is characterized by the absence of any viable microorganisms.
CSPs that must be sterile are, but not limited to, the following:
As seen on the table below, CSPs are distinguished into 2 categories based on the conditions under which they are made, their probability for microbial growth, and the time period allotted in which they must be used.
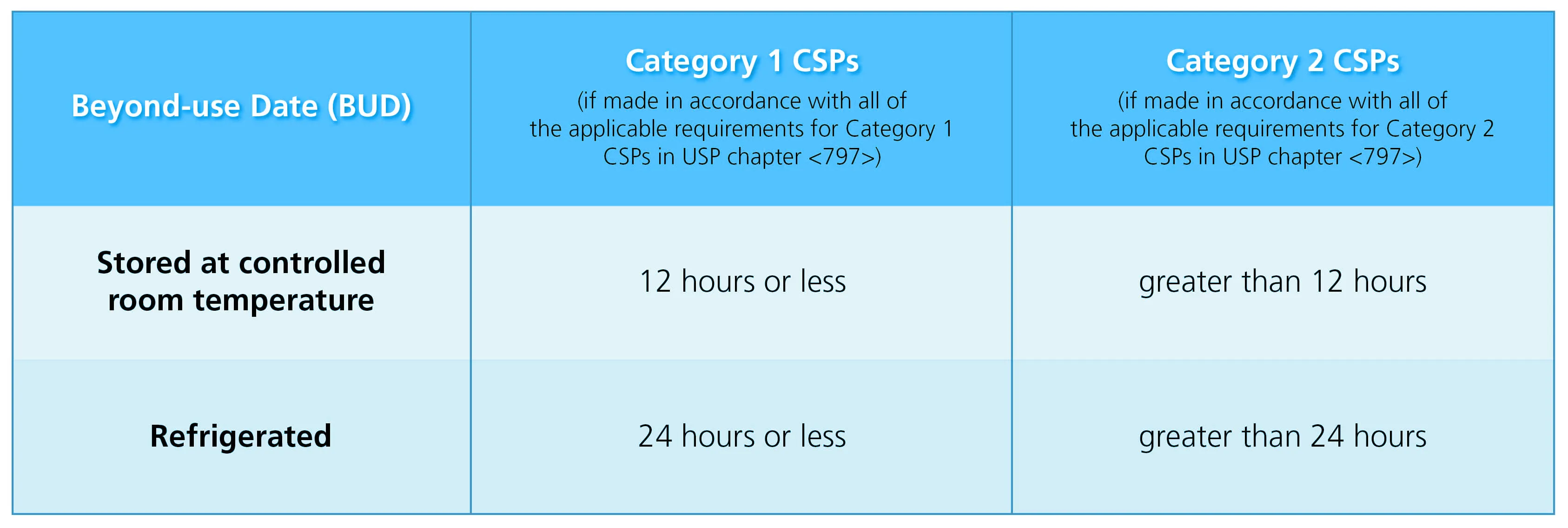 Table 1. Beyond-use dating of category 1 CSPs and category 2 CSPs
Table 1. Beyond-use dating of category 1 CSPs and category 2 CSPs
CSPs are compounded with the use of either sterile or non-sterile starting ingredients. When using sterile ingredient as the start-up component, its sterility must be maintained throughout the entire compounding process to achieve a CSP. While, when a non-sterile ingredient is used, it must first undergo sterilization, either by terminal sterilization in the final sealed container or through sterilizing filtration.
The quality of the components and the effectiveness of the sterilization step are critical to achieving a sterile preparation. Furthermore, aseptic technique must be utilized when preparing any sterile medication. Strict standard operating procedures (SOPs) must also be placed to minimize the potential for cross-contamination.
Sterile compounding facilities must be designed, outfitted, and maintained properly to minimize risk of contamination of CSPs. The design of the facility should take into account factors such as the number of operations being performed, the equipment (e.g., PECs, carts, computers), the personnel in the compounding area (and in adjacent areas), and the complexity of the compounding procedures; which are all considered as critical factors in maintaining control of environmental conditions in the facility. The required air quality within the facility must be achieved and maintained through primary engineering controls (PECs) and secondary engineering controls (SECs).
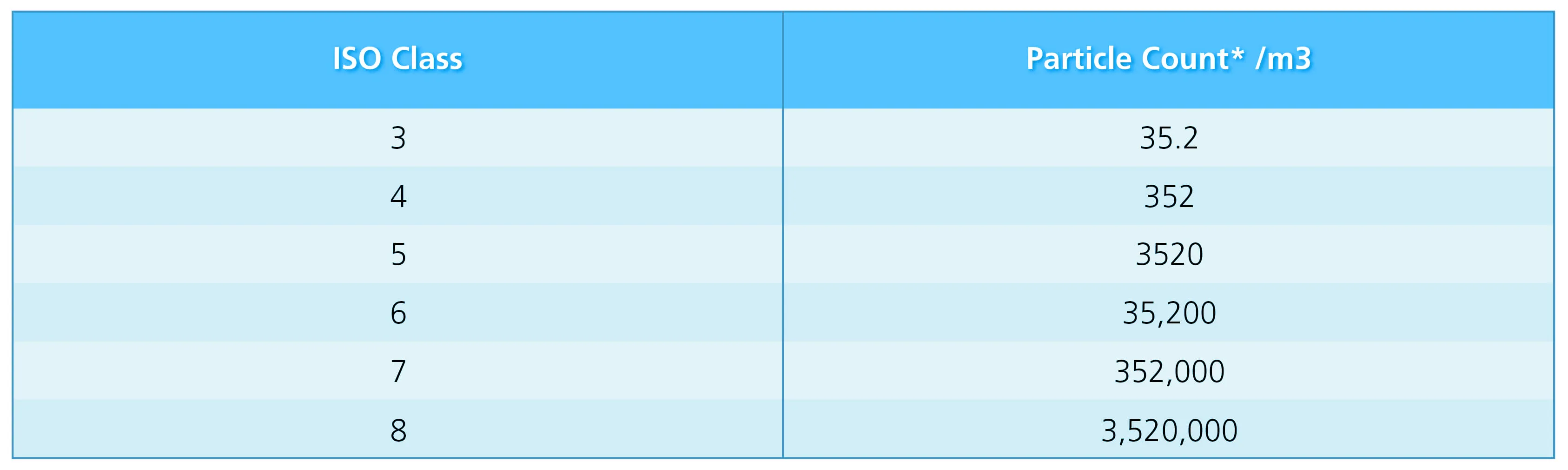 * Limits for number of particles ≥0.5 μm measured under dynamic operating conditions.
* Limits for number of particles ≥0.5 μm measured under dynamic operating conditions.
Table 2. ISO Classification of Particulate Matter in Room Air. From USP 42—NF 37: USP General Chapter <797> Pharmaceutical Compounding – Sterile Preparations (pg. 9), by United States Pharmacopeia, 2019, United States Pharmacopeial Convention.
Facilities used for compounding CSPs must be designed so that air quality improves with movement through separate operational areas, toward the PEC cleanroom. Classified areas where the air quality is controlled include ante-rooms, buffer rooms, and PEC work zone.
Primary engineering control (PEC) is defined as a device or zone that provides an ISO Class 5 air quality environment for sterile compounding. Category 1 and Category 2 CSPs must be prepared in a PEC that is certified to meet the ISO Class 5 or better conditions during dynamic operating conditions. PEC must be equipped with HEPA filters and a unidirectional airflow with a velocity sufficient to sweep particles from critical sites; this design prevents cross-contamination. HEPA-filtered air must be supplied by the PEC, and its air velocity must be sufficient to sweep away particles from critical sites and maintain a unidirectional airflow during operations.
Collectively, ante-rooms and buffer rooms are called secondary engineering controls. The secondary engineering control room (SEC) is defined as an area where the PEC is placed (e.g., a cleanroom suite or a segregated compounding area). It incorporates specific design and operational parameters required to minimize contamination risk within the compounding area.
Ante-rooms which provide access to positive pressure buffer rooms must at least meet the ISO Class 8 classification (see table 1 above); while, ante-rooms providing access to negative pressure buffer rooms must at least meet the ISO Class 7 classification. Typically, activities that potentially generate relatively higher levels of particulates are performed here (e.g., personnel hand hygiene, garbing procedures, and staging of components). The ante-room also serves as a transition area to ensure that proper air classification and pressure relationships are maintained between classified and unclassified areas in a facility.
On the other hand, buffer rooms must at least meet an ISO Class 7 air quality. Activities in this room must be controlled to prevent cross-contamination risks during the actual aseptic processing taking place in the PEC.
During occasions where only Category 1 CSPs will be compounded, the PEC may be placed in an unclassified area, without an ante-room or buffer room. This type of design is called a segregated compounding area (SCA). The impact of activities (e.g., patient care activities) that will be conducted around or adjacent to the SCA must be considered carefully, and a visible perimeter must be placed to establish the boundaries of the SCA.
Esco offers a wide range of products that are fit for the requirements of compounding CSPs. Examples of these include Isolators, Laminar Flow Straddle Units, and Cytoculture® Cytotoxic Safety Cabinets.
Esco also offers modular soft-walled and integrated cleanroom ceiling laminar airflow solutions which allow users to build cost-effective cleanroom suites. Moreover, maintenance of cleanroom condition is further enhanced by reducing cross-contamination through integration of pass boxes and air showers at the entrance and/or in between the controlled room and its external environment.
Through its four (4) core technologies, and along with its mission to create a safe industrial work environment, Esco Healthcare enables pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
References:
Recommended Products
Compounded non-sterile preparation (CNSP) is defined by the United States Pharmacopeia (USP) as a preparation intended to be non-sterile created by combining, admixing, diluting, pooling, reconstituting other than as provided in the manufacturer’s labeling, or otherwise altering of a drug or bulk drug substance.
Dosage forms that are considered as CNSPs include, but are not limited to, the following:
Facility requirements for CNSPs must ensure that equipment and materials are properly placed and arranged to prevent mix-ups among components, containers, labels, in-process materials, and finished products. The space should also be designed, arranged, and utilized in a manner that minimizes cross-contamination with non-compounding areas.
The equipment and supplies used for compounding a CNSP must be suitable for the specific compounding process. Disposable or dedicated consumables may also be used to reduce the chance of bioburden and cross-contamination. Equipment surfaces that come in contact with components must not be reactive, additive, or sorptive, and must not alter the quality of the CNSPs.
Equipment and devices used in the compounding or testing of compounded preparations must be inspected prior to its use and, if appropriate, be verified for accuracy. After compounding, the equipment must be cleaned to prevent cross-contamination of the next preparation.
Weighing, measuring, or otherwise manipulating components that could generate airborne chemical particles [e.g., active pharmaceutical ingredients (APIs), excipients, and conventionally manufactured products] must be assessed to determine the type of equipment suitable to handle each; like a closed system processing device to reduce the potential exposure to personnel or contamination of the facility or CNSPs. Examples of closed system processing devices include containment ventilated enclosures (CVEs), biological safety cabinets (BSCs), or single-use containment glove bags. The process evaluation shall be carried out in accordance with the facility SOP and be documented.
Esco offers a wide range of products that are fit for the requirements of compounding CNSPs. Examples of these include Isolators and Ventilated Balance Enclosures.
Esco also offers modular soft-walled and integrated cleanroom ceiling laminar airflow solutions which allow users to build cost-effective cleanroom suites. Moreover, maintenance of cleanroom condition is further enhanced by reducing cross-contamination through integration of pass boxes and air showers at the entrance and/or in between the controlled room and its external environment.
Through its four (4) core technologies, and along with its mission to create a safe industrial work environment, Esco Healthcare enables pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
References:
Recommended Products
Hazardous drugs (HDs) include those used for cancer chemotherapy, antiviral drugs, hormones, some bioengineered drugs, and other miscellaneous drugs.
HDs must be handled under conditions that promote patient/worker safety, and environmental protection. Signs designating the hazard must be displayed prominently before the entrance of HD handling areas.
Designated areas must be available for:
Certain areas are required to have higher negative pressure than the surrounding facility to contain HDs, and to minimize exposure risks. Engineering controls are required to protect the preparation from cross-contamination and microbial contamination (if preparation is intended to be sterile) throughout the compounding process. Engineering controls for containment are mainly divided into primary, secondary, and supplementary levels of control.
Containment primary engineering control (C-PEC) pertains to a ventilated device designed to minimize worker and environmental HD exposure during direct HD handling. While the containment secondary engineering control (C-SEC) refers to the room wherein which the C-PEC is placed. Lastly, the supplemental engineering controls [e.g., closed-system drug-transfer device (CSTD)] are adjunct controls recommended for additional levels of protection.
Sterile and nonsterile HDs must be compounded in a C-PEC that is located in a C-SEC that must:
Nonsterile hazardous compounding
When dealing with nonsterile hazardous compounding, C-PEC used must be externally vented (preferred) or have redundant-HEPA filters in series [e.g., Class I Biological Safety Cabinet (BSC), Contaminated Ventilated Enclosure (CVE) or compounding aseptic containment isolator (CACI)].
Since HD contamination is difficult to clean, surfaces of ceilings, walls, floors, fixtures, shelving, counters, and cabinets in the nonsterile compounding area must be smooth, impervious, free from cracks and crevices, and non-shedding.
A BSC o CACI being used for HDs compounding must not be used when preparing non-HDs unless the non-HD preparation is placed into a protective outer wrapper during removal from the C-PEC and is labeled to require PPE handling precautions.
For occasional non-sterile HD compounding, a C-PEC being used for sterile compounding (e.g., Class II BSC or CACI) may be used but must be decontaminated, cleaned and disinfected before resuming to any sterile compounding within the same C-PEC. A C-PEC is not required in cases where manipulation is limited to handling of final dosage forms (e.g., counting or repackaging of tablets and capsules) that do not generate particles, aerosols or gasses.
Sterile hazardous compounding
During sterile hazardous compounding, operations must be performed in a C-PEC that is externally vented, and capable of providing an ISO Class 5 or better air quality (e.g., Class II or III Biosafety Cabinets or CACI). The C-PEC must be located in a C-SEC, which may either be an ISO Class 7 buffer room with an ISO Class 7 ante-room (preferred) or an unclassified containment segregated compounding area (C-SCA); this may affect the beyond-use date (BUD) of the compounded sterile HD preparation.
When both non-sterile and sterile HDs are being compounded within an entity, the respective C-PECs must be placed in separate rooms; unless the C-PECs being used for non-sterile compounding are sufficiently effective that the room can continuously maintain ISO 7 classification throughout the non-sterile compounding activity.
If the C-PECs used for sterile and non-sterile compounding are placed in the same room, they must be placed at least 1 meter apart from each other, and particle-generating activity must not be performed while sterile compounding is in process.
Esco offers a wide range of products that are fit for handling and compounding of both sterile and nonsterile hazardous drugs. Examples of these include Isolators, Cytotoxic Safety Cabinet, and Ventilated Balance Enclosures.
Maintenance of controlled room condition and safety is enhanced with pass boxes, pass-through, and air showers at the exit, and/or in between the controlled room and its external environment.
Through its four (4) core technologies, along with its mission to create a safe industrial work environment for operator protection and product integrity, Esco Healthcare enables pharmaceuticals, nutraceuticals, and cosmeceuticals to comply with the internationally accredited GMP, as well as, industrial, environmental, and health and safety standards.
References:
Recommended Products
Sign up to our newsletter and receive the latest news and updates about our products!
