Large molecule drugs in the biopharmaceutical filed such as proteins and vaccines are administered in a parenteral/injectable route to directly reach the body’s systemic circulation. As such, they must be manufactured in a very aseptic/sterile conditions to remove the risk of microbial contamination which can easily degrade the product and cause unwanted adverse effects to the patients.
As per Annex 6 of the World Health Organization (WHO) good manufacturing practices for sterile pharmaceutical products:
“The production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks for personnel and/or for equipment and materials.
Clean areas should be maintained to an appropriate standard of cleanliness and supplied with air that has passed through filters of the required efficiency.”
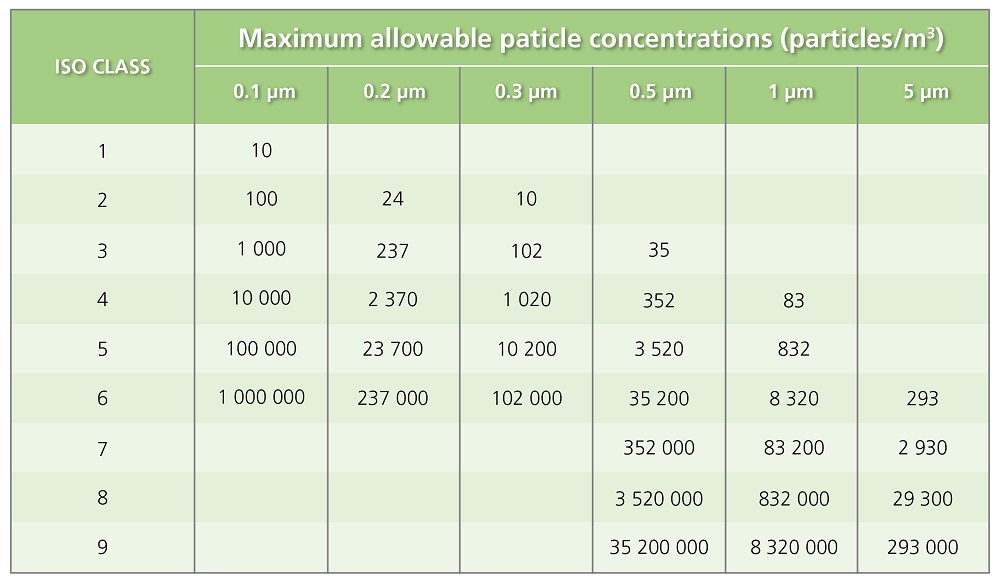
Table 1. US standards for Air Cleanliness classification follow ISO 14644-1 guidelines.
As per the United States Food and Drug Administration (USFDA) current good manufacturing guideline (cGMP), there are two different ways to manufacture sterile products:
- Terminal Sterilization
- This involves filling and sealing product containers under high-quality environmental conditions to minimize the microbial and particulate content of the in-process product. This helps ensure that the subsequent sterilization process is successful.
In its final container, the product is then subjected to a sterilization process such as heat or irradiation.
- Aseptic Processing
- In an aseptic process, the drug product, container, and closure are first subjected to sterilization methods separately, as appropriate, and then brought together.
There is no process to sterilize the product in its final container, so it is critical that containers be filled and sealed in an
extremely high-quality environment.
Aseptic processing involves more variables than terminal sterilization. Before aseptic assembly into a final product, the individual parts of the final product are subjected to various sterilization processes. Each of these processes require validation and control to prevent errors that could lead to the distribution of a contaminated product.
Any manual or mechanical manipulation of the sterilized drug, components, containers, or closures prior to or during aseptic assembly poses the risk of contamination and thus necessitates careful control.
Furthermore, as per the United States Pharmacopeia (USP) general chapter <797> in the production or preparation of sterile pharmaceuticals, the critical area where direct handling of the substance will be done, must be of an ISO Class 5 environment.
Advancements in Fill-Finish Operations
Pharmaceutical manufacturing involves complex and state-of-the-art process systems that have individually undergone strict validation tests for the assurance of repeatedly producing high quality products.
For more than half a century, pharmaceutical companies have been using an established industrial principle commonly known as ‘batch manufacturing’, which involves the use of an inconvenient number of individual equipment for each process. In fact, these units are so multifaceted that some of them are housed in an entirely separate Grade A environment; leaving a very large footprint. Running and maintaining these systems are highly expensive and not flexible enough to meet the fluctuating market demands.
Moreover, since this type of manufacturing is comprised of numerous discrete steps, the potential for human error is relatively high. To combat such problems agencies such as the US Food and Drug Administration (USFDA), have taken advantage of the recent technological advances in the field.
Continuous manufacturing (CM) refers to the concept of connected production; taking advantage of automation technology to link all operations together. This eliminates the possibility of delays in-between steps and allows the processed materials to move non-stop within the same facility. All the raw materials and other necessary manufacturing components are entered into a fully integrated assembly line; making it fast, flexible, and cost-effective.
Although moving from traditional to continuous manufacturing is fairly expensive, CM offers a promise of aseptic/sterile facilities with smaller footprint, lesser maintenance cost, and with a higher industrial profit.
In the manufacture of vaccines or other biopharmaceuticals, the best way to achieve such advanced manufacturing with lesser risk for microbial contamination and other sterility issues, is with the use of filling line systems.
A filling line is an integral part of manufacturing sterile pharmaceutical products. It ensures that parenteral medications are prepared inside an ISO classified environment for the whole manufacturing cycle, which is achieved through the use of a Restricted Access Barrier System (RABS) or an isolator (See
Esco Healthcare Ventures the Art of Filling Line Systems)
Esco Healthcare provides specialist services, equipment packages, and process solutions from our core platform products to cater the specific needs of each client. We are ready to meet your process needs and supply end-to-end solutions.
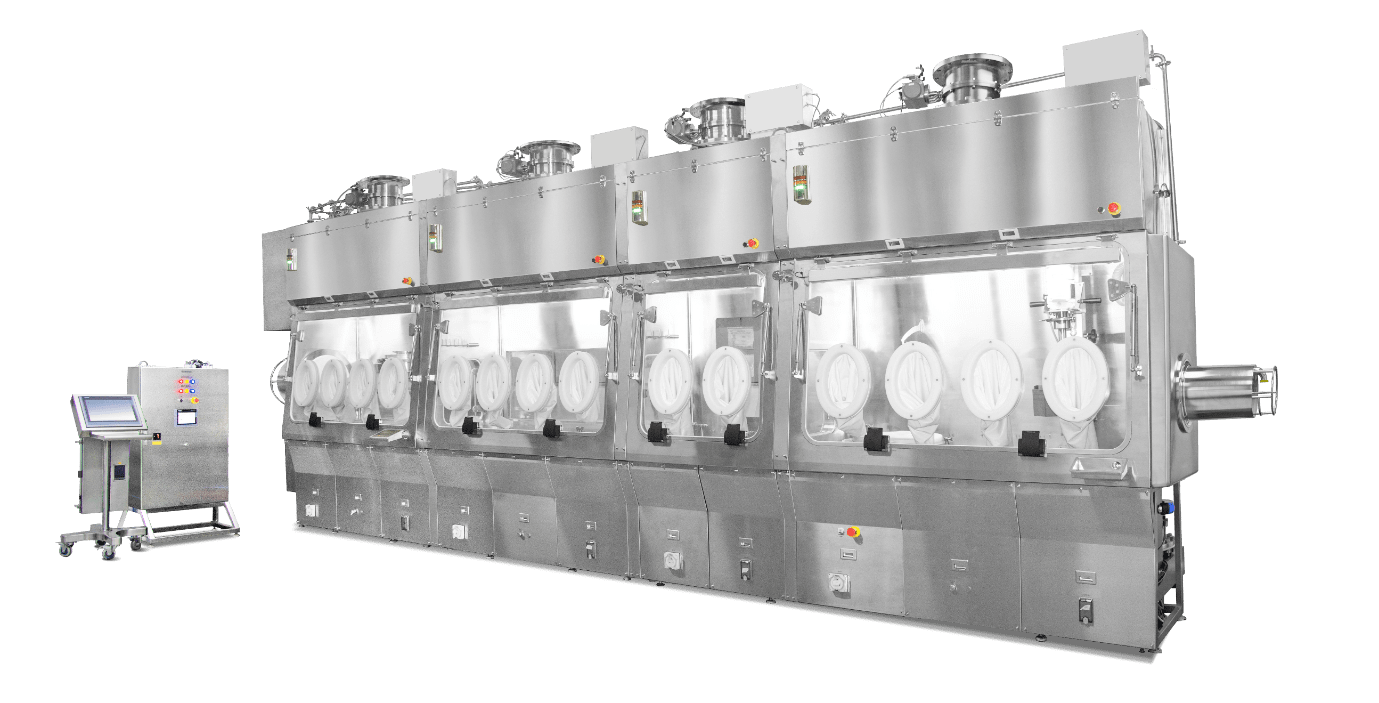
Figure 1. Formulation and filling line isolator.
Esco Healthcare's prized isolation technology can be configured at either positive or negative pressure with a recirculating or total exhaust unidirectional or turbulent airflow regime; depending on the process and the material to be handled by the clients. The isolator can house a whole filling line system for the production of sterile liquid and/or semi-solid pharmaceuticals. For materials with high occupational exposure bands (OEB), the necessary tools and equipment for powder handling can also be fully integrated inside. The components for the construction of this technology are modular and can be upgraded easily, very unlike traditional manufacturing.
Esco is equipped with the experience and the know-how to successfully integrate modular and innovative manufacturing technologies into your existing facilities; ensuring a safe and cost-effective way of advancing your processes.
At Esco Healthcare, we understand that each industry has its own unique requirements, and our products can be configured to meet each of them head-on.
Choose from Esco Healthcare's wide range of innovative core technologies and start your journey of modernized manufacturing with an established industrial partner.
References:
- ECA Academy. (2011). PIC/S Validation of Aseptic Processing (PI 007-6). Retrieved from: https://www.gmp-compliance.org/guidelines/gmp-guideline/pic-s-validation-of-aseptic-processing-pi-007-6-2011
- Mahmood, R. (2018). Good manufacturing practices for sterile pharmaceutical products. Journal of Developing Drugs, 7. doi: 10.4172/2329-6631-C2-028
- Pharmacy 180. (n.d.). Sterile Pharmaceutical Products - Introduction. Retrieved from: http://www.pharmacy180.com/article/sterile-pharmaceutical-products---introduction-646/
- U.S. Department of Health and Human Services. (2004). Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice. Food and Drug Administration. Retrieved from: https://www.fda.gov/media/71026/download
- U.S. Department of Health and Human Services. (2016). Pharmacy Compounding of Human Drug Products Under Section 503A of the Federal Food, Drug, and Cosmetic Act. Retrieved from: https://www.fda.gov/media/94393/download
- World Health Organization. (2011). WHO good manufacturing practices for sterile pharmaceutical products. Retrieved from: https://www.who.int/medicines/areas/quality_safety/quality_assurance/GMPSterilePharmaceuticalProductsTRS961Annex6.pdf
Recommended Products




