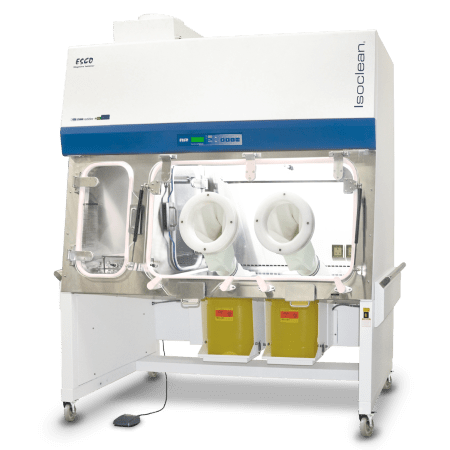
The Compounding Aseptic Containment Isolator (CACI) by Esco Healthcare is designed in compliance with the United States Pharmacopeia General Chapter <797> and <800> guidelines. It aims to provide a safe and clean environment for compounding of sterile hazardous drug preparations in.
Understanding CACI
Compounding Aseptic Containment Isolator (CACI) provides a safe and clean environment for compounding of hazardous, sterile drug preparations in compliance with USP 797 and 800 criteria. It is suitable for work involving hazardous materials, antineoplastic, or cytotoxic compounding applications.
The work zone and pass-through chambers are under negative pressure to maintain operator protection in case of a breach in the barrier isolation system. Clean air within the work zone must be supplied through a microbial retentive filter (HEPA minimum) system capable of containing airborne concentrations of the physical size and state of the drug being compounded.
During processes involving handling of volatile hazardous drugs, air must be externally vented from the isolator through a properly designed and dedicated building exhaust. This is the premium solution for every pharmacy’s compounding and containment needs.
Key Applications:
Potent Formulation
Aseptic Formulation
Sterile/Aseptic Compounding
Chemotherapy Compounding
Contained Powder Handling
Pharmacy Cytotoxic Compounding Isolator
Sub-division Isolator
Off-loading Containment Isolator
Advantages of Esco Healthcare’s CACI
Esco Healthcare’s Compounding Aseptic Containment Isolators (CACI) are designed with a standard HEPA (H14) filtration system with a 99.995% filtration efficiency at 0.1 to 0.3 microns, thus, providing an ISO Class 5 air quality in the chambers (as per ISO 14644-1). Options for a HEPA bag-in, bag-out (BIBO) exhaust filtration system is available for safe filtration change.
Pressure Regime
Negatively pressured and ISO 10648-2 Class 3 pressure leak tight CACI units, operate under a standard regime of -37 Pa in the work zone and -25 Pa in the pass-through chamber. This design ensures containment of the hazardous/toxic materials handled inside the isolator while also maintaining a sterile work zone to uphold the integrity and high quality of compounded sterile products (CSPs).
Airflow Regime
Esco Healthcare’s CACI units can be configured to have a recirculating or total exhaust/single pass airflow system. In a recirculating airflow regime, about 90% of HEPA-filtered air is recirculated within the isolator while approximately 10% of air is exhausted through such filters to prevent heat build-up in the system. The exhausted air will then be replenished by ambient air coming from the top in-let G4 pre-filters with 80% efficiency. This airflow regime can be recommended for compounding facility’s not handling volatile hazardous drugs, especially for institutions that do not have the capacity for a 100% exhaust/ducting system.
However, as per USP <800> guidelines, for facility’s handling volatile hazardous drugs/substances it is better to have a total exhaust/single pass equipment that will be connected to a ducting/exhaust system. It must be understood that HEPA filters are for the removal of particles (0.1 to 0.3micron sizes) and are not designed for filtration of gases or volatile materials. As such, the sure way to fully remove the volatile materials from the isolator and from the facility is to duct the isolator to a dedicated building exhaust system. The airflow for this total exhaust/single pass isolator ensures that all air passing through the supply HEPA filters will also be exhausted out through a series of HEPA exhaust filter system (option for BIBO).
Each compounding facility has its own protocols for various processes, and understanding the needs of each process is critical in choosing the correct equipment that will help protect the actual sterile compounded product as well as the operator and the environment from untoward exposure or cross-contamination.
References:
- USP Compounding Expert Committee, (nd). General Chapter <797> Pharmaceutical Compounding – Sterile Preparations. Retrieved from: https://www.usp.org/compounding/general-chapter-797 [Accessed 11 November 2020].
- USP Compounding Expert Committee, (nd). General Chapter <800> Hazardous Drugs – Handling on Healthcare Settings. [ONLINE] available at: http://www.usp.org/sites/default/files/usp/document/our-work/healthcare-quality-safety/general-chapter-800.pdf. [Accessed 11 November 2020].
Related Products