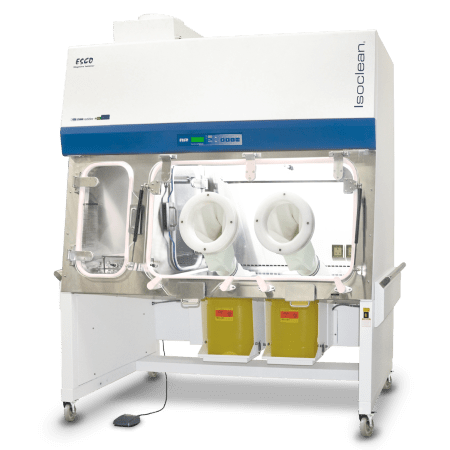
Isoclean® Healthcare Platform Isolator - Inflatable Seal Model (HPI-IS)
Esco Healthcare’s Isoclean® Healthcare Platform Isolator – Inflatable Seal (HPI-IS) model guarantees the full isolation of a product or process inside an ISO Class 5 or Grade A environment, for high-quality final products.
Along with its inflatable seals and automated dampers, HPI-IS is equipped with an automated pressure hold testing (APHT) and mobile BioVap™ biodecontamination system.
This model can be factory-configured to operate in either a positive or negative pressure mode, with a recirculating or single pass/total exhaust airflow scheme.
Applications:
Aseptic and/or Potent Compounding
Pharmacy Compounding
Sterility Testing
Cell and Gene Therapy
Peptide Production
Biosafety Facility Level 3 or 4
Pressure-tested as per ISO 10648-2:
selection from three (3) material of constructions:
- Class 2 pressure-leak tight enclosure via auto pressure leak test (factory acceptance test)
- Class 3 via automated pressure test as per ISO 10648-2 (prior to daily tasks)
Superior ISO Class 5 Air Filtration
ULPA filters (as per IEST-RP-CC001.3 and HEPA (H14) filter (as per EN 1822) with a typical efficiency of > 99.999% at 0.1 to 0.3 microns; provide superior ISO Class 5 air cleanliness as per ISO 14644-1.
Automated pressure hold testing (APHT)
APHT provides a smooth way of quantifying the hourly leak rate of the isolator, thus, ensuring the unit’s integrity against leaks while removing the risk for cross-contamination.
Integrated BioVap™ biodecontamination system with H2O2 sensors and catalytic converter.
- Integration of the Biovap™ biodecontamination system allows ease of decontaminating the work chambers with just a press of a button in the Esco HMI.
- HPI-IS with integrated BioVap™ allows master biodecontamination (only performed with internal doors open).
- Master biodecontamination procedure refers to the possibility to biodecontaminate the entire isolator inner chambers (process chamber and pass-through chambers) with a H2O2 mist.
Automated sliding door feature
for closing and opening of the inner pass-through chamber door.
- Facilitates ease of isolation control especially during pressure decay testing and biodecontamination process.
Inflatable seal and automated dampers:
for closing and opening of the inner pass-through chamber door.
- For an improved and safer isolation control during pressure decay testing and bio-decontamination process.
- HPI-IS automated dampers remove the need to manually close/open up the isolator’s dampers every time the unit needs to undergo pressure testing and biodecontamination cycle.
Esco HMI control system
supervises all cabinet operations and monitors cabinet performance in real-time.
Electromagnetic interlocking doors with time delay effect
ensures safety and containment during material transfer.
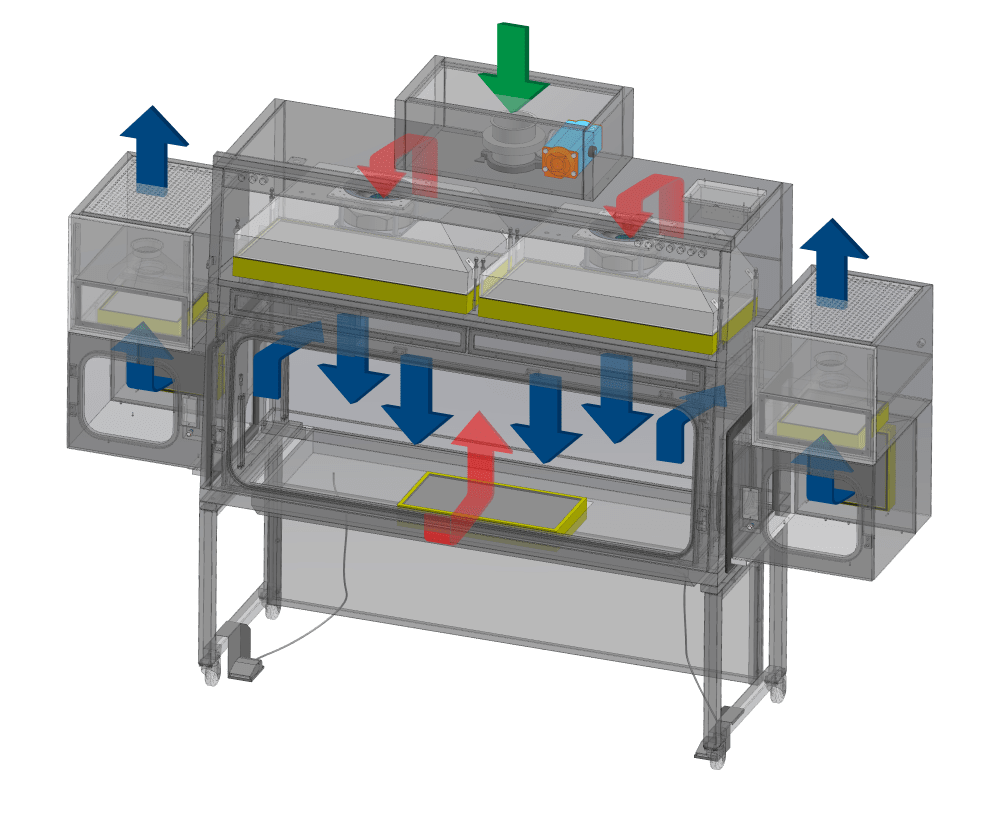
Positive Pressure, Recirculating Model
Ambient air is pulled through the inlet pre-filter and main filter located on top of the isolator. The HEPA (H14) downflow filter creates a laminar air flow providing ISO Class 5 air cleanliness (ISO 14644-1) to the main chamber.
A percentage of the air from the work zone is recirculated back to the chamber. The fan pulls the purged air back to the plenum which again passes through the HEPA (H14) downflow filters, resulting to the air being recirculated back to the work zone and pass-through.
Meanwhile, a percentage of air is pulled towards the perforations at the rear wall inside the work zone, which is then transferred to the pass chamber via the network of HEPA (H14) filters located at its own rear walls. The filtered air is then exhausted out through the top portion of the PTC after passing through another stage of HEPA (H14) filters.
A percentage of purged air is exhausted through the filters to prevent heat build-up inside the isolator. Exhausted air will then be replenished by ambient air coming from the top inlet pre-filter and the main filter.
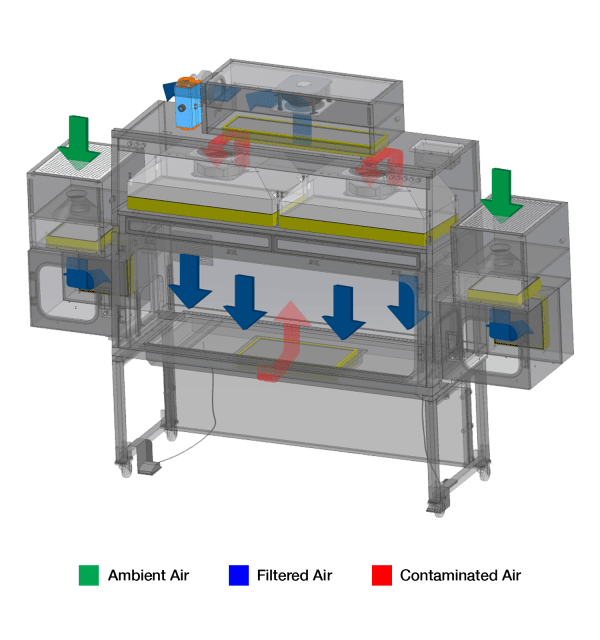
Negative Pressure, Recirculating Model
Ambient air is pulled through the inlet pre-filter and HEPA (H14) filter located on top of the pass-through chambers of the isolator. The air is then pulled by the fan towards the rear wall of the PTC and goes to the back plenum.
It passes through the HEPA (H14) downflow filter again, resulting to the air being recirculated to the work zone.
The HEPA (H14) downflow filter creates a laminar air flow providing ISO Class 5 air cleanliness (ISO 14644-1) to the main chamber. Exhausted air is then replenished by ambient air coming from the top inlet pre-filter and the main filter.
|
Isoclean® Healthcare Platform Isolator – Inflatable Seal Model |
|||||||||||||||
|
Isolator Unit |
Model |
No. of Gloves |
Voltage Code |
Pressure |
Airflow |
Pass-through Chamber |
Sharps Bin Inside |
||||||||
|
HPI |
Isoclean® Healthcare Platform Isolator |
IS |
Inflatable Seal |
2G |
2 Gloves |
8 |
220-240 VAC, 50/60 Hz |
N |
Negative |
S |
Single-Pass/ Total Exhaust |
0 |
No PTC |
S |
with Sharps Container inside main chamber |
|
3G |
3 Gloves |
9 |
110-120 VAC, 50/60 Hz |
P |
Positive |
R |
Recirculating |
L |
1 PTC Left |
O |
Without Sharps Container |
||||
|
4G |
4 Gloves |
R |
1 PTC Right |
||||||||||||
|
B |
1 PTC Both sides |
||||||||||||||
-
Available in Negative or Positive Pressure model, in Recirculating or Total Exhaust airflow
- Positive pressure is designed for process/product protection.
- Negative pressure is intended for containment purposes where the operator and environmental protection are necessary.
- Recirculating airflow scheme: designed for dealing with non-hazardous and sterile materials.
- Single Pass or Total Exhaust airflow scheme: recommended when handling volatile (hazardous) substances.
- Integration of Esco mobile BioVap™ biodecontamination system (H2O2 biodecontamination with sensor and catalytic converter)
- Integration of a side-mounted 24L CO2 Incubator
- Glove Leak Tester
-
Glove Leak sizes
- - 300mm
- - 300 x 200 mm
- CCTV integration
- Height adjustable stand
- Access to rear view monitor system
- With option for three-way pass through chamber in between 2 units of 2-glove, 3-glove, or 4-glove isolator combination
-
Other configurations available:
- 2-glove + 2-glove + 2-glove units
- 4-glove + 2-glove units
- NEBB Cleanroom Performance Testing for Validation
- Optional PQ Support