Containment Barrier Isolator: The Practical Solution to cGMP Compliance

The year 2019 has been a season of success for Esco Healthcare - Esco’s division that provides quality equipment and promotes a safe working environment to the healthcare industry. This year, the division released a recent addition to its range of Containment Barrier Isolator (CBI) which paved the way for more applications and processes to be carried out in a cost-effective yet cGMP-compliant equipment.
The CBI technology facilitates the isolation of a product or a process while providing the required conditions for a sterile/aseptic and hazardous environment. This full stainless-steel isolator provides a comprehensive range of personnel, product, and environmental protection.
Standard Features of the CBI
Full stainless-steel isolator with a stainless steel 304 exterior and a fully-welded stainless steel 316L internal chambers with rounded coved corners
Class 2 Leak Tight Containment, as per ISO 10648-2 standards
Esco HMI control system supervises all functions and provides real-time monitoring of cabinet status
Safe change glove system for the maintenance of aseptic conditions inside the chamber during glove/sleeve replacement
Airlock pass-through chamber maintains work zone sterility during material transfer
Foot switch Provides hands-free opening of the magnetic interlock minimizing operator fatigue during transfer procedures.
FDA-grade static seals
Four Models of CBI
-

CBI - Unidirectional (CBI-U)
CBI-U utilizes a unidirectional/laminar airflow and facilitates the isolation of a product or process while providing the required conditions (ISO Class 5/Grade A Environment) for a sterile/aseptic environment.
Models Available:
-
Positive or Negative Pressure
-
Recirculating or Total Exhaust/Single-Pass Airflow
-
2-,3-, or 4-gloves
Common Applications:
Pharmacy compounding (Chemotherapy/Total Parenteral Nutrition)
Cell processing
Aseptic processing
Sterility testing
Medical device manufacturing
Radiopharmacy
Cosmeceutical
Nutraceutical
Food and beverage application
Research and development
-
-

CBI - Turbulent (CBI-T)
CBI-T utilizes turbulent airflow and facilitates product or process isolation while providing the required condition for handling potent powders. CBI-T may come with static seals (CBI-T-SS) or inflatable seals (CBI-T-IS).
Models Available:
-
Negative Pressure only
-
Total Exhaust/Single-Pass Airflow
-
2-,3-, or 4-gloves
Common Applications:
Potent powder handling
HPAPI QC Testing
Research and development
-
-

CBI - Class III Biosafety Cabinet (CBI-III)
CBI-III offers the highest level of operator, product, and environmental protection from infectious/biohazardous aerosols and is suitable for microbiological work with agents assigned to biosafety levels 1, 2, 3, or 4. It is designed for an absolute level of containment - frequently used for work involving the deadliest biohazards, bacteria, viruses, and other microorganisms.
Models Available:
-
Negative Pressure only
-
Total Exhaust/Single-Pass Airflow
-
2-,3-, or 4-gloves
Common Applications:
Biosafety Levels 1 to 4 handling
Virus production
Vaccine production
-
-

CBI - Hybrid (Class III/Class I Convertible Biosafety Cabinet (CBI-H)
CBI-H builds on Esco Pharma’s success of CBI-III. It allows operators to work via a removable glove visor to convert the cabinet and function as a Class III or a Class I Biosafety Cabinet as per EN 12469 standards.
In BSC Class III mode, the operator works through a glove port attached to a removable panel. It can be converted to a BSC Class I by removing the closure panel and attaching a blanking plate over the inlet HEPA filter.
Models Available:
-
Convertible Class III to Class I BSC
-
2,3, or 4 gloves
Common Applications:
Biosafety Levels 1 to 4 handling
Virus production
Vaccine production
-
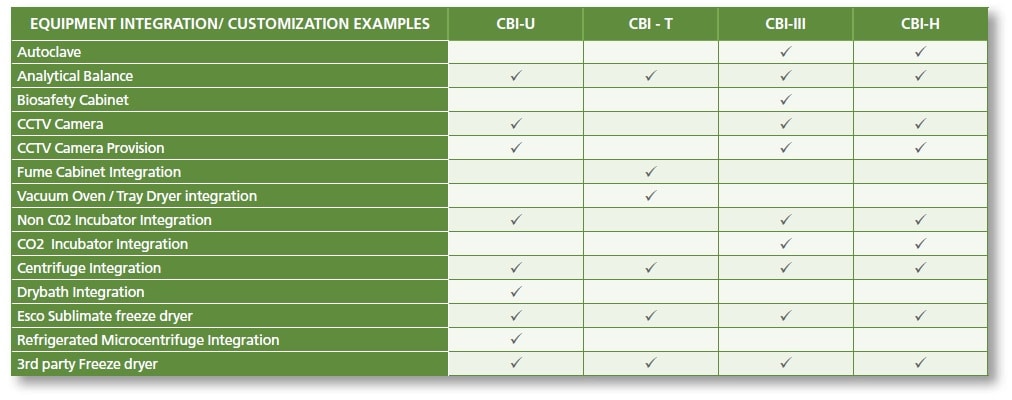
Equipment Integration
Depending on the process requirements, the different models of CBI can be integrated with multiple equipment for an easier and a more efficient workflow. CBI offers the advantage of equipment integration without hampering the containment of the cabinet. Equipment can be integrated into the work zone, main chamber walls, or even into pass-through chamber walls, which will be defined by the process and the application.
Testing and validation of each equipment will be meticulously carried out to ensure the isolator’s performance comply with international standards, despite the design integrations.
LIST OF EQUIPMENT INTEGRATION OPTIONS
Esco Healthcare continues to innovate and create state-of-the-art technologies that promote safe working environment for the healthcare sector.
As 2019 bids adieu, the company continues to look forward to more opportunities for growth and industry-wide collaborations.
For more information about the CBI, click on the link below:
/products-sub-page.php?productId=40&pg=pdtDownload CBI Brochure






