Protection Guaranteed with Esco Healthcare Turnkey Solutions

Operator Protection
Containing hazardous particulates for the overall protection of operators

Product Protection
Preventing cross-contamination to promote product quality integrity.

Environmental Protection
Preventing industry-wide exposure against hazardous materials.

Clean Air
Provides ISO classified air quality for the aseptic manufacturing of sterile products.
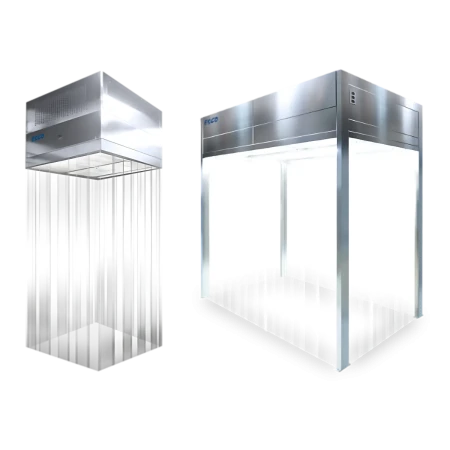
Airflow Containment
Airflow containment technology generally provides laminar airflow, making it suitable for large-scale industrial applications. Some products are designed to give operator and environment protection from hazardous substances, while others are designed to give product protection by providing an area with specified ISO Class 5 or Grade A environment.
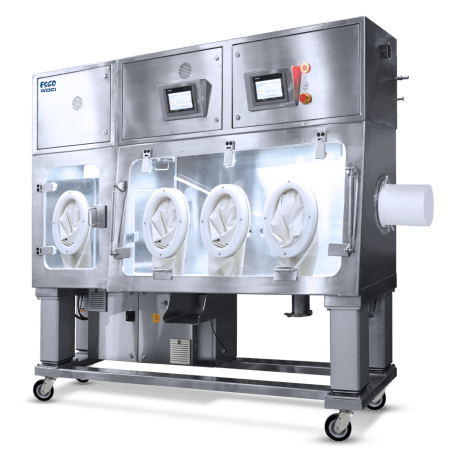
Cross-Contamination Facility Integrated Barrier
Cross-contamination facility integrated barrier (CCFIB) technology ensures that all matter entering and exiting the cleanroom are purged off of any contaminants; preventing events for cross-contamination.
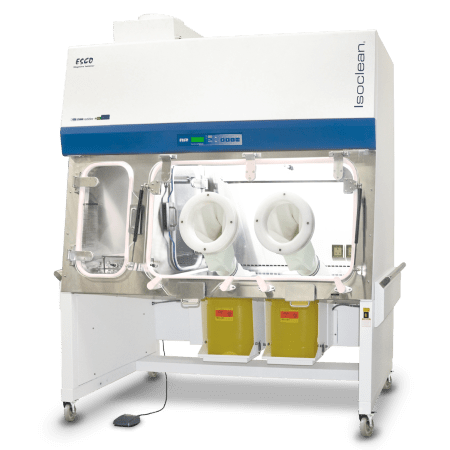
Isolation Containment
Isolation containment technology provides an isolated work zone via a pressure-tight enclosure to ensure absolute separation between the operator and the product/process. Isolators provide clean air to the workzone and enable ISO Class 5 as per ISO14644-1 (Grade A as per EU GMP) or better environment for aseptic/sterile processing.
Ventilation Containment
Ventilation containment technology ensures high level of operator protection, and facilitates stability and accuracy, when handling non-sterile hazardous substance(s) such as hazardous airborne powders.