For years radiolabelled blood cells played a critical role as diagnostic tracers especially for cardiac blood pool imaging or thrombus detection. Studies suggested that components of thrombotic and fibrinolytic systems are able to carry radiolabels to a specific thrombi. In recent years, new methods have been studied including the improved method of conducting platelet labelling.
Key characteristic of a radiotracer used for blood cell labelling is its ability to easily bind to target cells (i.e. thrombi) and of course, its rapid blood disappearance rate. These two ensure provision of a clear patient diagnosis within a few hours.
Clinical Indications of Blood Cell Labelling:
- Measurement of total red blood cell (RBC) volume
- Measurement of RBC survival time
- Identification of RBC destruction sites
- Blood pool imaging studies (i.e. gastrointestinal bleeding)
- Selective spleen imaging with damaged RBCs
99mTc Labelled Red Blood Cells (RBCs)
Using the isotope of technetium-
99m (
99mTc) to label red blood cells in nuclear cardiology is well established. This isotope is able to efficiently distribute itself inside the body’s intravascular pool and subsequently, able to leave it at a slow rate. This timeframe allows practitioners to collect high resolution images with the use of a gamma scintillation camera. Even information on regional myocardial wall motion and left ventricular ejection fraction can be obtained.
If this isotope is used in its pertechnetate form (TcO4-) it can mimic the movement and distribution characteristics of iodine, and as such, concentrates into the thyroid and salivary glands, gastric mucosa, and even the choroid plexus (network of blood vessels in each ventricle of the brain). However, because of this characteristic, this radioisotope is not firmly bound to RBCs and will easily diffuse into the extravascular fluid compartment, and accumulate in aforementioned organs. Thus, it is important that the
99mTc is firmly bound to the cells and will persist in vivo during the observation period.
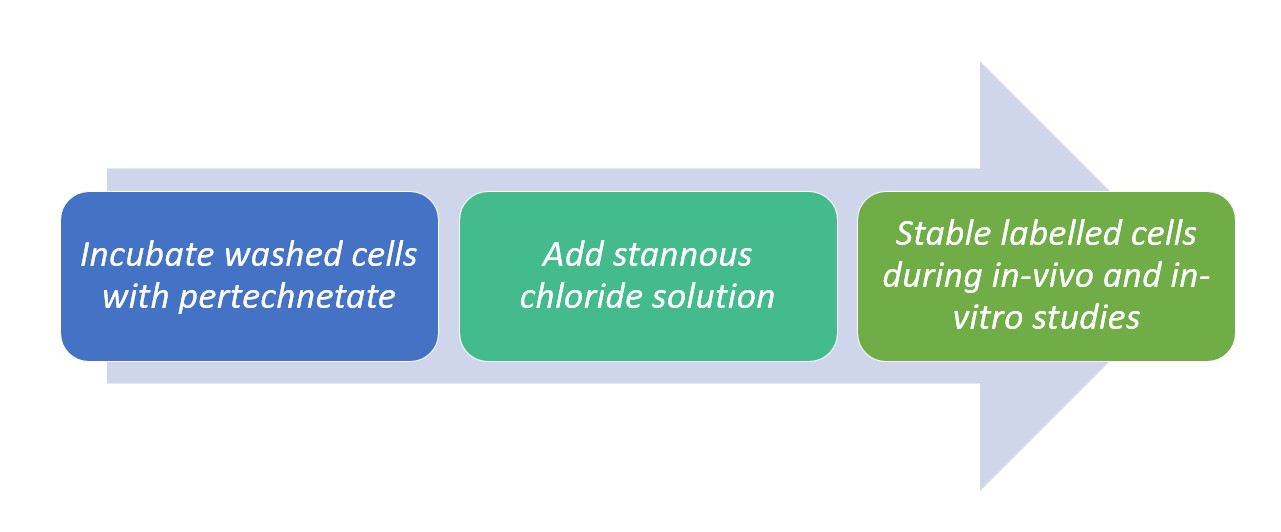
Studies established a 99mTc labelling method with 50 to 60% efficiency reports through the use of stannous chloride as a reducing. The method is as follows:
Figure 1. Method of labelling 99mTc with SnCl2
Actual red blood cell labeling with
99mTc occurs whenever
99mTc pertechnetate is brought into contact with RBCs previously treated with stannous ions. This can be accomplished by either the in vivo or in vitro addition of Tc-
99m pertechnetate to RBCs that have been pre-treated with stannous ions.
It must be noted tohough that even if it is technetium in the +7 (pertechnetate) oxidation state that crosses the intact erythrocyte membrane, only technetium reduced to a lower oxidation state will firmly bind to hemoglobin. This is where the importance of stannous chloride comes in as normally, stannous ions are often employed for the reduction of technetium.
Stannous ions hydrolyses and precipitates at physiologic pH, so in vivo and modified in vivo methods are done via direct intravenous administration.
Moreover, in order to reduce the concentration of extracellular stannous ions, biological clearance must be observed. There should be a 20 to 30-minute allowance in between the injection of the stannous solution and the administration of the
99mTc. Previous practice suggests the use of centrifugation in order to remove the extracellular stannous ions.
Labelling Methods with 99mTc RBC:
- In Vitro Kits – simplified the labelling process of 99mTc radioisotopes with stannous citrate and glucoheptonate by eliminating the multiple washing steps previously employed, along with removing the need to extemporaneously and aseptically compound said stannous substances (for intravenous (IV) injection).
A major advantage brought by said kits is the ability to prepare reagents in advance and store them.
- In Vivo Method: labelling is accomplished with two subsequent IV injections – first with the non-radioactive stannous solution and followed by the 99mTc pertechnetate.
- Modified In-Vivo Method: generally evolved from the above technique with a measurable rate of incorporation into RBCs. During labelling and after a sufficient period, pretinned RBCs and 99mTc pertechnetate are isolated from body compartments. The blood labelled cells will then have 90% of the total technetium isotope tightly bound to the RBCs, resulting to an increased intravascular retention and therefore, an improved image quality.
References:
- https://www.sciencedirect.com/science/article/abs/pii/S000129980580176X
- https://pharmacyce.unm.edu/nuclear_program/freelessonfiles/vol1lesson3.pdf
- Saha, G.B., 2004. Fundamentals of Nuclear Pharmacy. 5th edition. New York: Springer
- Do, K.H., 2016. General Principles of Radiation Protection in Fields of Diagnostic Medical Exposure. Journal of Korean Medical Science [epub 2016 Jan 26]
- UK Radiopharmacy Group, 2009. Guidelines for the Safe Preparation of Radiolabelled Blood Cells.
- Radiological Society of North America, Inc. 2016. General Nuclear Medicine. https://www.radiologyinfo.org/en/info.cfm?pg=gennuclear . Accessed: 11 July 2017
- European Association of Nuclear Medicine (EANM) Radiopharmacy Committee, 2007. Guidelines on Current Good Radiopharmacy Practices (cGRPP) for Radiopharmaceuticals in Nuclear Medicine. Available at: http://www.eanm.org/scientific_info/guidelines/gl_radioph_cgrpp.pdf
- European Association of Nuclear Medicine (EANM) Technologists Committee, 2008. The Radiopharmacy: A Technologist’s Guide.
- International Atomic Energy Agency, 1979. Technical Report Series No. 194: Preparation and Control of Radiopharmaceuticals in Hospitals. Vienna, Austria.
Recommended Products



