

Discovery to Delivery
› Solutions ›Enteral
Oral Solid Dosage Forms
A. Dispensing Tablets
B. Hypodermic Tablets
These tablets are formed by the process of pressing powdered, crystalline or granular materials, alone or in combination with excipients to form a compact adherent mass of predetermined shape.
Factors Influencing the Variety of Compressed Tablets
I. The manner of use of the tablet, such as:
II. Specific mode of action
I. The manner of use of the tablet
Chewable Tablets
Buccal Tablets
Sublingual Tablets
Implantation Tablets or Pellets
Vaginal Tablets, Tablet Suppositories or Inserts

Multiple Layered Tablets
Layered Tablets
Press-coated Tablets
Sugar-coated Tablets

Film-coated Tablets
Advantages of Special Oral Tablets:
Advantages of Compressed Tablets:
Properties of a Compressed Tablet
The tablet is a vehicle for conveying the medication to the patient and therefore, the formulator should be aware of the limitations of the raw materials and equipment since these affect the bioavailability of the medicament.
Tableting Machine
Basic parts of Tableting Machines

Types of Tableting Machines
This is consist of a hopper, feed shoe, weight adjustment, collar (which when turned clockwise, reduces the fill weight and when turned counter clockwise increases the fill weight), one set of the cavity and punches (upper and lower), discharge chute, cam and an upper adjustment collar (which determines the position of the lower punch at the point of ejection of the finished tablet)
For increase production output, these machines offer great advantages. A head carrying a number of sets of dies and punches revolves continuously, while the tablet granulation runs from the hopper, through a feed frame and into the dies placed in a large steel plate revolving around it.
The rotary tablet machines have been developed into models capable of producing multiple-layered tablets. These machines are able to make one, two or three layered tablets.
Preparation of Tablet Components for Compression
I. Dry Granulation Method
— Also called Pre-compression; Double Compression Method
— This method is applicable to tablet ingredients that are/ have sensitive to moisture
Raw material ➔ weighing ➔ Screening ➔ Mixing ➔ Slugging ➔ Milling ➔ Screening ➔ Mixing ➔ Compression
A. Direct Compression
Raw material ➔ Weighing ➔ Screening ➔ Mixing ➔ Compression
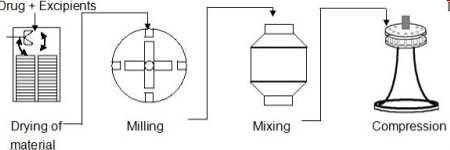
B. Granulation by Compression
This method is adopted for tablets components that are sensitive to heat, moisture or both. It is further divided into two methods namely
II. Wet Granulation Method
A. Wet Granulation
Disadvantages
Raw material ➔ Weighing ➔ Screening ➔ Wet massing ➔ Sieving/Milling ➔ Drying ➔ Screening ➔ Mixing ➔ Compression
Tablet Additives and Components
Additives or Excipients furnish bulk or volume to the tablets and are classified according to their functions as follows:
1. Diluents
These are substances that make up the major portion of the tablet, usually:
1.a. Lactose USP
Most often used because of the following advantages:
1.b. Starches

1.c. Sucrose
1.d. Mannitol
1.e. Microcrystalline Cellulose (Avicel)
1.f. Hydrolyzed starch with dextrose
Other common diluents
2. Binders
These Excipients are substances that “glue” powders together, and cause them to form granules. Binders are added in dry form to the powdered medicaments and activated by the addition of water or other solvents to form a slurry or paste.
Examples of raw materials employed as binders are:
3. Disintegrants
These are substances or agents added to compressed tablets to cause them to “break apart” or disintegrate, when placed in an aqueous medium.
Examples of raw materials employed as disintegrants are:
Super disintegrants: Swells up to ten fold within 30 seconds when contact water.

4. Lubricants
These are substances which:
Examples of raw materials employed as lubricants are:
5. Coloring Agents
Coloring agents are added to tablet granulations for the following purposes:
6. Flavoring Agents
These are substances added to tablet formulations mainly to “mask” the undesirable flavor of certain medicaments.
Examples of raw materials employed as flavoring agents:
Sweetening agents
7. Adsorbents
These are substances capable of holding quantities of fluids or moisture in an apparently dry state.
Examples of raw materials employed as adsorbents:
Sugar coating machine
Sugar coating machines are used for average coating and polishing of formed tablets and pills in pharmaceutical and food industry.
WORKING PRINCIPLE
Other Application:
In film coating:
Benefits of Sugar Coating Machine
STAGES INVOLVED IN THE PRODUCTION OF SUGAR COATED TABLETS

SEALING
Tablet Sealants
Subcoating
2 Methods of Subcoating
Smoothing or Grossing
Color-coating
— traditional method; process is prone to coating faults eg, poor and uneven coverage, and variation in color from batch to batch
— Easier to use and permit comparatively fast color coating
Polishing
Printing
Methods of Coating syrup application
Traditional panning equipment, capable of achieving impressive results in skilled hands but this technique is not easy to control in terms of modern GMP requirements
These control devices allow programmed variation in dose volume, rolling time, and drying time
Other equipment having efficient drying ability



Ideal Characteristics of Sugar coated tablets
specifications and any appropriate compendial requirements
Film Coating
Film Coating Machine
Characteristics:
The machine adopt advanced design concept, has compact structure, meet cGMP standard completely.
Process Description
Coating Solution Formulation
POLYMER
Other Polymers
Enteric coating polymers
SOLVENT
Modern techniques rely on water as a solvent because of the significant drawbacks that readily became apparent due to the use of organic solvents.
Solvents used for coating:
PLASTICIZERS
COLORANTS
Supercell™ Tablet Coater
Advantages
Capsules are solid dosage forms in which the drug is enclosed within either a hard or soft soluble container or “shell”.
They are usually formed from gelatin; however, they also may be made from starch or other suitable substances.
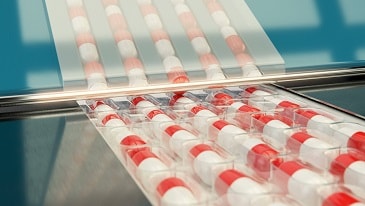
HARD SHELL CAPSULES
Capsule sizes and capacity
Capsule |
Range of powder capacity |
|---|---|
| No. 5 | 60-130mg |
| No. 4 | 95-260mg |
| No. 3 | 130-390mg |
| No. 2 | 195-520mg |
| No. 1 | 225-650mg |
| No. 0 | 325-910mg |
| No. 00 | 390-1300mg |
| No. 000 | 650-2000mg |
Types of Gelatin Capsules
Method of Production of the Hard Gelatin Capsules
The “Pin Method” of production is adapted, with the use of completely automatic machines
MECHANISMS:
Proper Storage of Empty Hard Gelatin Capsules:
Finishing Filled Hard Gelatin Capsules
Pan Polising
— polyurethane or cheeseclothCloth Dusting
— The bulk filled capsules are rubbed with a cloth (may or may not be impregnated with oil)Brushing
— rotating soft brushes/vacuumSpecial Techniques for Hard Gelatin Capsules
Utilize for the following reasons:
Pharmaceutical Application of Soft Gelatin Capsules
Method of Production of Gelatin Capsules
A. PLATE PROCESS
B. ROTARY DIE PROCESS
C. RECIPROCATING DIE PROCESS
A. Plate Process
The oldest, commercial method
Steps of Plate Process:B. Rotary Die Process
A continuous process of production of Soft Gelatin Capsules, invented by Scherer in 1993.
C. Reciprocating Die Process
Developed in 1949
Steps of Reciprocating Die Process:MICROENCAPSULATION
— a process in which tiny particles or droplets are surrounded or coated with a continuous film of polymeric material to give more useful properties.
Importance of Microencapsulation
Microencapsulation produces
CAPSULE STANDARD
Uniformity of Fill Weight Test
— Simple means of estimating content of active ingredient per capsule.
Suspensions may be defined as preparations containing finely divided drug particles (the suspensoid) distributed somewhat uniformly throughout a vehicle in which the drug exhibits a minimum degree of solubility. Some suspensions are available in ready-to-use form, that is, already distributed through a liquid vehicle with or without stabilizers and other additives.

Features Desired in a Pharmaceutical Suspension
Physical Features of the Dispersed Phased of a Suspension
In most good pharmaceutical suspensions, the particle diameter is 1 to 50μm. Generally, particle size reduction is accomplished by dry milling prior to incorporation of the dispersed phase into the dispersion medium. One of the most rapid, convenient, and inexpensive methods of producing fine drug powders of about 10 to 50μm size is micropulverization. Micropulverizers are high-speed attrition or impact mills that are effi cient in reducing powders to the size acceptable for most oral and topical suspensions. For still finer particles, under 10μm, fluid energy grinding, sometimes referred to as jet milling or micronizing, is quite effective. By this process, the shearing action of high-velocity compressed airstreams on the particles in a confined space produces the desired ultrafine or micronized particles. The particles to be micronized are swept into violent turbulence by the sonic and supersonic velocities of the airstreams. The particles are accelerated to high velocities and collide with one another, resulting in fragmentation. This method may be employed when the particles are intended for parenteral or ophthalmic suspensions. Particles of extremely small dimensions may also be produced by spray drying. A spray dryer is a cone-shaped apparatus into which a solution of a drug is sprayed and rapidly dried by a current of warm, dry air circulating in the cone. The resulting dry powder is collected. It is not possible for a pharmacist to achieve the same degree of particle-size reduction with such comminuting equipment as the mortar and pestle.
Variety of dosage forms maybe found packaged in plastic containers, such as:
The disadvantages of plastic packaging
Metal Containers
Disperse systems having a consistency of a soft paste, gel, cream, or ointment can be conveniently packed into “collapsible tubes”, which are commonly made of:
Rubber Components
Rubber of varying composition is used in pharmaceuticals and biologicals as:
Problems encountered in the use of rubber closures:
Manufacturing Considerations in Liquids
The rate at which the equilibrium is achieved, is highly dependent in the details of the following:
SUSPENSIONS
These liquids are heterogeneous systems consisting of two phases:

Methods of Preparation of Suspensions
A. By discipitation method
B. By precipitation method
I. Discipitation Method
This process is done by dispersing the finely divided powders in an appropriate liquid vehicle.
The use of surfactants is desirable to ensure uniform wetting of the hydrophobic surfaces.
II. By Precipitation Methods
This is performed by effecting precipitation in the liquid vehicle.
A. Organic Solvent Precipitation
— Water insoluble drugs are precipitated by dissolving them in a water miscible organic solvent. Then adding the organic phase to purified water under standard conditions.
B. Precipitation effected by changing the pH
— This is applicable to those drugs in which its solubility is dependent on pH value.
C. By Double Decomposition Method
— Where simple chemistry is involved.
— Example is the preparation of White Lotion USP (made by interacting zinc sulfate with sulfurated potash solution to form zinc polysulfide).
Other points to be considered in preparing suspensions
3. Incompatibilities
— Some incompatibilities that may occur in suspensions are:
Emulsion may be defined in a number of ways, but essentially, they are “heterogeneous system, usually containing two immiscible liquids”.
Purposes of emulsions
Types of Emulsions
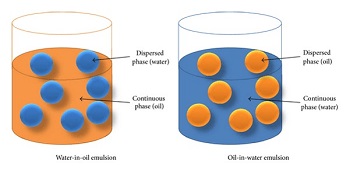
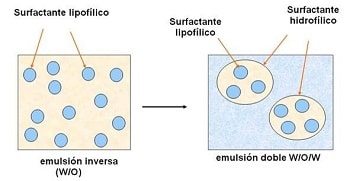
General Considerations of Emulsification
| TYPE OF TESTS | W/O | O/W |
|---|---|---|
| A. Dye solubility Test | Amaranth green (-)
Sudan red (+) |
(+)
(-) |
| B. UV Flourescence Test | (+) absorption | (-) |
| C. Conductivity | (-) | (+) |
| D. Cobalt Chloride Test | Blue | Pink |
| E. Direction of Creaming | Sedimentation | Creaming |
Dry gum (o + e) + w
Addition of gum to oil (mixing lightly to disperse), followed by adding all water and stir continuously & vigorously. Continuous trituration for further 2-3 minutes to produce white stable emulsion.
— The whiter the product, the smaller the globules. Other ingredients should be added gradually up to its final volume.
Wet gum (w + e) + o
Water is added to the gum and quickly triturated until gum has dissolved, to make mucilage. Oil is then added to mucilage in small proportions, triturating after each addition, until a thick primary emulsion is obtained.
Syrups are concentrated aqueous preparations of a sugar or sugar substitute with or without flavoring agents and medicinal substances. Syrups containing flavoring agents but not medicinal substances are called non medicated or flavored vehicles (syrups)
These syrups are intended to serve as pleasant tasting vehicles for medicinal substances to be added in the extemporaneous compounding of prescriptions or in the preparation of a standard formula for a medicated syrup, which is a syrup containing a therapeutic agent.
Due to the inability of some children and elderly people to swallow solid dosage forms, it is fairly common today for a pharmacist to be asked to prepare an oral liquid dosage form of a medication available in the pharmacy only as tablets or capsules. In these instances, drug solubility, stability, and bioavailability must be considered case by case.
Medicated syrups are commercially prepared from the starting materials, i.e., by combining each of the individual components of the syrup, such as sucrose, purified water, flavoring agents, coloring agents, the therapeutic agent, and other necessary and desirable ingredients. Naturally, medicated syrups are employed in therapeutics for the value of the medicinal agent present in the syrup.
Syrups provide a pleasant means of administering a liquid form of a disagreeable-tasting drug. They are particularly effective in the administration of drugs to youngsters, since their pleasant taste usually dissipates any reluctance on the part of the child to take the medicine. The fact that syrups contain little or no alcohol adds to their favor among parents
Components of Syrups
Most syrups contain the following components in addition to the purifi ed water and any medicinal agents present: (a) the sugar, usually sucrose, or sugar substitute used to provide sweetness and viscosity; (b) antimicrobial preservatives; (c) fl avorants; and (d) colorants. Also, many types of syrups, especially those prepared commercially, contain special solvents, solubilizing agents, thickeners, or stabilizers.
Sucrose- and Nonsucrose-based Syrups
Sucrose is the sugar most frequently employed in syrups, although in special circumstances, it may be replaced in whole or in part by other sugars or substances such as sorbitol, glycerin, and propylene glycol. In some instances, all glycogenetic substances (materials converted to glucose in the body), including the agents mentioned earlier, are replaced by nonglycogenetic substances, such as methylcellulose or hydroxyethylcellulose. These two materials are not hydrolyzed and absorbed into the blood stream, and their use results in an excellent syrup-like vehicle for medications intended for use by diabetic patients and others whose diet must be controlled and restricted to nonglycogenetic substances. The viscosity resulting from the use of these cellulose derivatives is much like that of a sucrose syrup. The addition of one or more artifi cial sweeteners usually produces an excellent facsimile of a true syrup.
Preparation of Syrup
Syrups are most frequently prepared by one of four general methods, depending on the physical and chemical characteristics of the ingredients. Broadly stated, these methods are (a) solution of the ingredients with the aid of heat, (b) solution of the ingredients by agitation without the use of heat, or the simple admixture of liquid components, (c) addition of sucrose to a prepared medicated liquid or to a flavored liquid, and (d) percolation of either the source of the medicating substance or the sucrose.
Solution with the Aid of Heat
Syrups are prepared by this method when it is desired to prepare the syrup as quickly as possible and when the syrup’s components are not damaged or volatilized by heat. In this method, the sugar is generally added to the purified water, and heat is applied until the sugar is dissolved. Then, other heat-stable components are added to the hot syrup, the mixture is allowed to cool, and its volume is adjusted to the proper level by the addition of purified water. If heat-labile agents or volatile substances, such as volatile flavoring oils and alcohol, are to be added, they are generally added to the syrup after the sugar is dissolved by heat, and the solution is rapidly cooled to room temperature.
The use of heat facilitates rapid solution of the sugar and certain other components of syrups; however, caution must be exercised against becoming impatient and using excessive heat. Sucrose, a disaccharide, may be hydrolyzed into monosaccharides, dextrose (glucose), and fructose (levulose). This hydrolytic reaction is inversion, and the combination of the two monosaccharide products is invert sugar. When heat is applied in the preparation of a sucrose syrup, some inversion of the sucrose is almost certain. The speed of inversion is greatly increased by the presence of acids, the hydrogen ion acting as a catalyst to the reaction. Should inversion occur, the sweetness of the syrup is altered because invert sugar is sweeter than sucrose, and the normally colorless syrup darkens because of the effect of heat on the levulose portion of the invert sugar. When the syrup is greatly overheated, it becomes amber colored as the sucrose caramelizes. Syrups so decomposed are more susceptible to fermentation and to microbial growth than the stable, undecomposed syrups. Because of the prospect of decomposition by heat, syrups cannot be sterilized by autoclaving. The use of boiled purified water in the preparation of a syrup can enhance its permanency, and the addition of preservative agents, when permitted, can protect it during its shelf life. Storage in a tight container is a requirement for all syrups.
Solution by Agitation without the Aid of Heat
To avoid heat-induced inversion of sucrose, a syrup may be prepared without heat by agitation. On a small scale, sucrose and other formulative agents may be dissolved in purified water by placing the ingredients in a vessel larger than the volume of syrup to be prepared, permitting thorough agitation of the mixture. This process is more time consuming than the use of heat, but the product has maximum stability. Huge glass lined or stainless steel tanks with mechanical stirrers or agitators are employed in large-scale preparation of syrups.
Sometimes simple syrup or some other non-medicated syrup, rather than sucrose, is employed as the sweetening agent and vehicle. In that case, other liquids that are soluble in the syrup or miscible with it may be added and thoroughly mixed to form a uniform product. When solid agents are to be added to a syrup, it is best to dissolve them in minimal amount of purified water and incorporate the resulting solution into the syrup. When solid substances are added directly to a syrup, they dissolve slowly because the viscous nature of the syrup does not permit the solid substance to distribute readily throughout the syrup to the available solvent and also because a limited amount of available water is present in concentrated syrups.
Addition of Sucrose to a Medicated Liquid or to a Flavored Liquid
Occasionally a medicated liquid, such as a tincture or fluidextract, is employed as the source of medication in the preparation of a syrup. Many such tinctures and fluidextracts contain alcohol-soluble constituents and are prepared with alcoholic or hydro alcoholic vehicles. If the alcohol-soluble components are desired medicinal agents, some means of rendering them water soluble is employed. However, if the alcohol-soluble components are undesirable or unnecessary components of the corresponding syrup, they are generally removed by mixing the tincture or fluidextract with water, allowing the mixture to stand until separation of the water-insoluble agents is complete, and filtering them from the mixture. The filtrate is the medicated liquid to which the sucrose is added in preparation of the syrup. If the tincture or fluidextract is miscible with aqueous preparations, it may be added directly to simple syrup or to a flavored syrup.
Percolation
In the percolation method, either sucrose may be percolated to prepare the syrup or the source of the medicinal component may be percolated to form an extractive to which sucrose or syrup may be added. This latter method really is two separate procedures: first the preparation of the extractive of the drug and then the preparation of the syrup.
An example of a syrup prepared by percolation is ipecac syrup, which is prepared by adding glycerin and syrup to an extractive of powdered ipecac obtained by percolation. The drug ipecac, which consists of the dried rhizome and roots of Cephaëlis ipecacuanha, contains the medicinally active alkaloids emetine, cephaeline, and psychotrine. These alkaloids are extracted from the powdered ipecac by percolation with a hydroalcoholic solvent.
The syrup is categorized as an emetic with a usual dose of 15 mL. This amount of syrup is commonly used in the management of poisoning in children when evacuation of the stomach contents is desirable. About 80% of children given this dose will vomit within half an hour. For a household emetic in the event of poisoning, 1-oz. bottles of the syrup are sold without a prescription. Ipecac syrup also has some application as a nauseant expectorant in doses smaller than the emetic dose.
Evidence indicates that many bulimics— most commonly young women in their late teens to early 30s—use the syrup of ipecac to bring on attacks of vomiting in an attempt to lose weight . Pharmacists must be aware of this misuse of the syrup of ipecac and warn these individuals because one of the active ingredients in the syrup is emetine. With continual use of the syrup, emetine builds up toxic levels within the body tissues and can do irreversible damage to the heart muscles in 3 to 4 months, resulting in symptoms mimicking a heart attack. Shortness of breath is the most common symptom in patients who misuse the syrup of ipecac, but some persons describe low blood pressure– related symptoms and irregularities of heartbeat.
Elixirs are clear, sweetened hydroalcoholic solutions intended for oral use and are usually flavored to enhance their palatability. Non medicated elixirs are employed as vehicles, and medicated elixirs are used for the therapeutic effect of the medicinal substances they contain. Compared with syrups, elixirs are usually less sweet and less viscous because they contain a lower proportion of sugar and consequently are less effective than syrups in masking the taste of medicinal substances. However, because of their hydro alcoholic character, elixirs are better able than aqueous syrups to maintain both water soluble and alcohol-soluble components in solution. Also, because of their stable characteristics and the ease with which they are prepared (by simple solution), from a manufacturing standpoint, elixirs are preferred to syrups.
The proportion of alcohol in elixirs varies widely because the individual components of the elixirs have different water and alcohol solubility characteristics. Each elixir requires a specific blend of alcohol and water to maintain all of the components in solution. Naturally, for elixirs containing agents with poor water solubility, the proportion of alcohol required is greater than for elixirs prepared from components having good water solubility. In addition to alcohol and water, other solvents, such as glycerin and propylene glycol, are frequently employed in elixirs as adjunctive solvents.
Although many elixirs are sweetened with sucrose or with a sucrose syrup, some use sorbitol, glycerin, and/or artificial sweeteners. Elixirs having a high alcoholic content usually use an artificial sweetener, such as saccharin, which is required only in small amounts, rather than sucrose, which is only slightly soluble in alcohol and requires greater quantities for equivalent sweetness.
All elixirs contain flavorings to increase their palatability, and most elixirs have coloring agents to enhance their appearance. Elixirs containing more than 10% to 12% of alcohol are usually self-preserving and do not require the addition of an antimicrobial agent.
Preparation of Elixirs
Elixirs are usually prepared by simple solution with agitation and/or by admixture of two or more liquid ingredients. Alcohol-soluble and water-soluble components are generally dissolved separately in alcohol and in purified water, respectively. Then the aqueous solution is added to the alcoholic solution, rather than the reverse, to maintain the highest possible alcoholic strength at all times so that minimal separation of the alcohol-soluble components occurs. When the two solutions are completely mixed, the mixture is made to the volume with the specified solvent or vehicle. Frequently, the final mixture will be cloudy, principally because of separation of some of the flavoring oils by the reduced alcoholic concentration. If this occurs, the elixir is usually permitted to stand for a prescribed number of hours to ensure saturation of the hydroalcoholic solvent and to permit the oil globules to coalesce so that they may be more easily removed by filtration. Talc, a frequent filter aid in the preparation of elixirs, absorbs the excessive amounts of oils and therefore assists in their removal from the solution. The presence of glycerin, syrup, sorbitol, and propylene glycol in elixirs generally contributes to the solvent effect of the hydroalcoholic vehicle, assists in the dissolution of the solute, and enhances the stability of the preparation. However, the presence of these materials adds to the viscosity of the elixir and slows the rate of filtration.
Types of Elixirs
Non medicated elixirs may be useful to the pharmacist in the extemporaneous filling of prescriptions involving (a) the addition of a therapeutic agent to a pleasant-tasting vehicle and (b) dilution of an existing medicated elixir. In selecting a liquid vehicle for a drug substance, the pharmacist should be concerned with the solubility and stability of the drug substance in water and alcohol. If a hydro alcoholic vehicle is selected, the proportion of alcohol should be only slightly above the amount needed to effect and maintain the drug’s solution. When a pharmacist is called on to dilute an existing medicated elixir, the non -medicated elixir he or she selects as the diluent should have approximately the same alcoholic concentration as the elixir being diluted. Also, the flavor and color characteristics of the diluent should not be in conflict with those of the medicated elixir, and all components should be chemically and physically compatible. In years past, when pharmacists were called on more frequently than today to compound prescriptions, the three most commonly used non-medicated elixirs were aromatic elixir, compound benzaldehyde elixir, and isoalcoholic elixir.
Most official and commercial elixirs contain a single therapeutic agent. The main advantage of having only a single therapeutic agent is that the dosage of that single drug may be increased or decreased by simply taking more or less of the elixir, whereas when two or more therapeutic agents are present in the same preparation, it is impossible to increase or decrease the dose of one without an automatic and corresponding adjustment in the dose of the other, which may not be desired. Thus, for patients required to take more than a single medication, many physicians prefer them to take separate preparations of each drug so that if an adjustment in the dosage of one is desired, it may be accomplished without the concomitant adjustment of the other.
Tinctures are alcoholic or hydroalcoholic solutions prepared from vegetable materials or from chemical substances. They vary in method of preparation, strength of the active ingredient, alcoholic content, and intended use in medicine or pharmacy. When they are prepared from chemical substances (e.g., iodine, thimerosal), tinctures are prepared by simple solution of the chemical agent in the solvent.

Depending on the preparation, tinctures contain alcohol in amounts ranging from approximately 15% to 80%. The alcohol content protects against microbial growth and keeps the alcohol-soluble extractives in solution. In addition to alcohol, other solvents, such as glycerin, may be employed. The solvent mix of each tincture is important in maintaining the integrity of the product. Tinctures cannot be mixed successfully with liquids too diverse in solvent character because the solute may precipitate. For example, compound benzoin tincture, prepared with alcohol as the sole menstruum, contains alcohol-soluble principles that are immediately precipitated from solution upon addition of water.
Because of the alcoholic content, tinctures must be tightly stoppered and not exposed to excessive temperatures. Also, because many of the constituents found in tinctures undergo a photochemical change upon exposure to light, many tinctures must be stored in light-resistant containers and protected from sunlight.
Medicated tinctures taken orally include Paregoric, USP, or camphorated tincture of opium. Usually, patients requiring oral medication nowadays prefer to take a tablet or capsule or a pleasant-tasting elixir or syrup. Tinctures have a rather high alcoholic content, and some physicians and patients alike prefer other forms of medication. Opium Tincture, USP, or laudanum, is much more potent than paregoric, and the two should not be confused. Any prescription for either one should be carefully evaluated and the dose checked and confirmed.
Aromatic waters are clear, aqueous solutions saturated with volatile oils or other aromatic or volatile substances. Aromatic waters are no longer in widespread use. In years past, aromatic waters were prepared from a number of volatile substances, including orange flower oil, peppermint oil, rose oil, anise oil, spearmint oil, wintergreen oil, camphor, and chloroform. Naturally, the odors and tastes of aromatic waters are of the volatile substances from which they are prepared. Most of the aromatic substances in the preparation of aromatic waters have very low solubility in water, and even though the water may be saturated, its concentration of aromatic material is still rather small. Aromatic waters may be used for perfuming and/or flavoring.

Spirits are alcoholic or hydro alcoholic solutions of volatile substances. Generally, the alcoholic concentration of spirits is rather high, usually over 60%. Because of the greater solubility of aromatic or volatile substances in alcohol than in water, spirits can contain a greater concentration of these materials than the corresponding aromatic waters. When mixed with water or with an aqueous preparation, the volatile substances present in spirits generally separate from the solution and form a milky preparation. Spirits may be used pharmaceutically as flavoring agents and medicinally for the therapeutic value of the aromatic solute. As flavoring agents, they are used to impart the flavor of their solute to other pharmaceutical preparations. For medicinal purposes, spirits may be taken orally, applied externally, or used by inhalation, depending upon the particular preparation. When taken orally, they are generally mixed with a portion of water to reduce the pungency of the spirit. Depending on the materials, spirits may be prepared by simple solution, solution by maceration, or distillation. The spirits most recently official in the USP–NF are aromatic ammonia spirit, camphor spirit, compound orange spirit, and peppermint spirit.
Sign up to our newsletter and receive the latest news and updates about our products!
