

Discovery to Delivery
› Solutions ›Eye care/ Ophthalmic preparations
Ophthalmic preparations are sterile products that may contain one or more pharmaceutical ingredient (s) administered topically, or by subconjunctival or intraocular (e.g. intravitreal and intracameral) injection in the form of solution, suspension, or ointment. Ophthalmic formulations also require that the pH, buffer capacity, viscosity, and tonicity is carefully controlled. These may include anesthetics, antibiotics, antioxidants, antivirals, cataract therapies, corticosteroids, decongestants, miotics, lubricants, and macular degeneration and nutritional support formulas.
Strict adherence to aseptic technique and proper sterilization procedures are crucial in the preparation of ophthalmic products. All extemporaneous compounding of ophthalmic products must be prepared in the proper sterile environment, with adherence to USP <797> standards and performance of necessary sterility testing to ensure quality and safety of products.
The compounding of ophthalmic preparations requires strict adherence to sterility requirements to ensure the safety of patients. Sterility is an absolute condition that is essential in manufacturing these products, as any contamination can cause harm to the eyes. Manufacturers must comply with current Good Manufacturing Practice (cGMP) guidelines and other regulations to ensure the sterility of ophthalmic preparations.
Improper sterile compounding of eye preparations can compromise their sterility, leading to serious consequences when used on human beings. Ophthalmic preparations are directly administered to the eye tissue, including the conjunctiva or eyelid, which are vulnerable to infection due to the mucous membrane. If foreign substances from the eye preparations are introduced, it can cause infections such as conjunctivitis, as shown in Figure 1 below. This can result in long-term loss of sight and other critical complications.
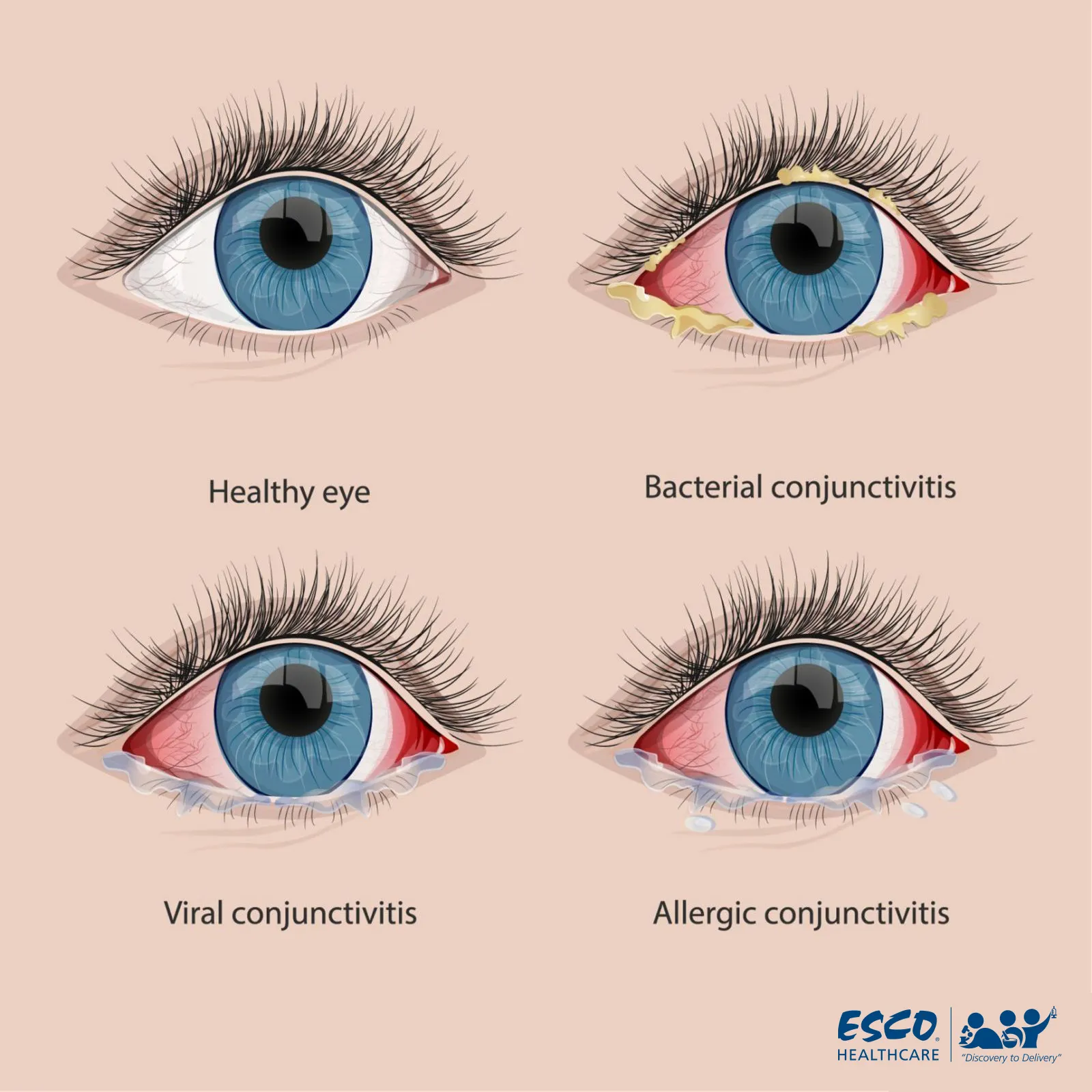
Fig. 1 Comparison of Healthy Eyes and Conjunctivitis
The compounding of ophthalmic preparations requires the use of aseptic techniques to ensure that the products are free of microorganisms. Aseptic techniques involve methods to minimize contamination from medical devices or personnel, thereby reducing exposure to the products.
To achieve an aseptic environment, a separate clean area with Laminar Airflow (LAF) and air filtration using HEPA filters or the building's HVAC system is required. Other helpful equipment includes a Pass Box for transferring materials from the gray area to the clean area, as well as equipment to monitor pressure and temperature.
Terminal sterilization for eye preparations is typically achieved through solution filtration with a membrane filter, such as with eye drops. This is an important step to ensure sterility in ophthalmic preparations.
Sterility testing of eye preparations is carried out in an aseptic environment to ensure that the products are sterile. This testing is crucial to confirm the efficacy of the aseptic techniques used during the compounding process.
Maximizing the efficacy of ophthalmic mixtures involves several important concepts, such as adjusting the pH and osmolarity of the solution, ensuring the formulation's sterility, and optimizing drug solubility in the solution.
In general, the pH of the solution should closely resemble the physiologic pH, although there is a limited range of acceptable pH values that are nearest to the physiologic pH. The osmolarity of the solution must also be adjusted to ensure that it is isotonic with the tears and does not cause irritation.
Sterility is also crucial in ophthalmic formulations, and a preservative is typically used to prevent the growth of microorganisms in the product. This is an important step to ensure that the product remains safe and effective for use.
Overall, these concepts are critical to ensuring that ophthalmic mixtures are safe and effective for use in treating ocular conditions. By optimizing the pH and osmolarity of the solution, ensuring sterility, and improving drug solubility, ophthalmic formulations can be maximized for therapeutic benefit.
Eye preparations are often used to treat ocular inflammation symptoms of eye disorders and diseases. These preparations are typically administered topically, as many drugs such as proteins, peptides, and chemotherapeutic agents are rendered inactive when they reach the gastrointestinal tract if taken orally. Additionally, the bioavailability of ocular drugs is known to be very low compared to oral drugs due to protective mechanisms in the eyes that limit drug penetration.
Eye drops and eye ointments are two popular types of eye preparations available on the market. Eye drops are made of soluble drugs in a solution, while eye ointments are made of an oil base that disperses the drugs. As previously mentioned, eye ointments have low drug penetration for eye drop products. However, eye ointments can overcome this issue by having longer contact with the conjunctiva.
| Classification | Eye drops | Eye ointments |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Furthermore, research and application of sophisticated drug delivery systems for eye preparation are becoming increasingly common in order to increase drug penetration. The studies progressed from the Traditional Ocular Drug Delivery System (DDS) to the Novel Ocular DDS, then to the Control Delivery System, and finally to the Advanced Delivery System.
Emulsions, suspensions, and ointments are examples of traditional DDS. Novel DDS also includes nanotechnology, liposomes, dendrimers, and niosomes. Implants, iontophoresis, contact lenses, collagen shield, ocular inserts, and micro-needles are all scoped by the Control Delivery System. Cell encapsulation, stem cell therapy, oligonucleotide therapy, and other therapies are available through the Advanced Delivery System. The conclusion of the level of drug delivery system of ocular preparations is shown in Figure 2.
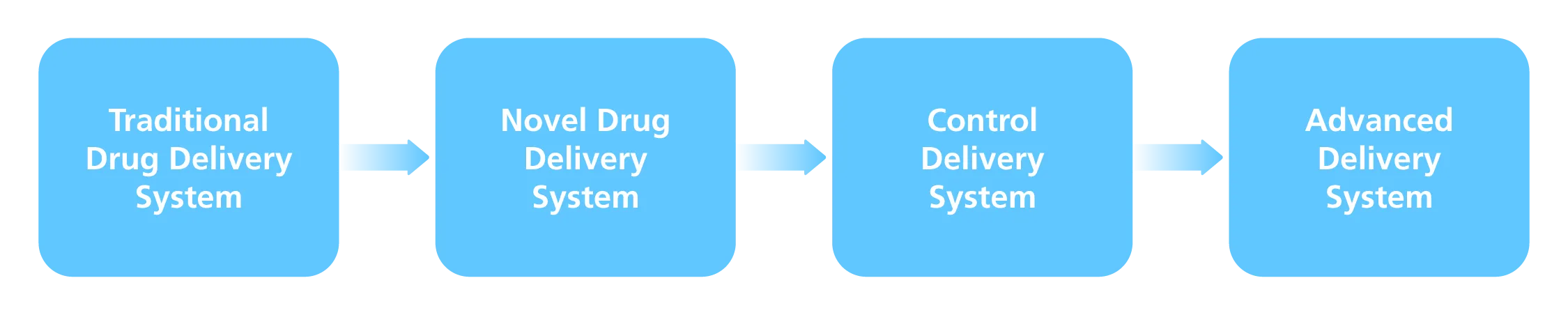
Fig. 2 Drug Delivery System Level of Ocular Preparation
Ophthalmic preparations such as eye drops and ointments are used by countless individuals around the world. Even the World Health Organization (WHO) is actively addressing the issue of eye disorders and leveling the knowledge regarding treatment. Thus, to create safe ophthalmic products and ensure effectiveness, manufacturers must have appropriate facilities, particularly the sterility testing to ensure quality, safety, and efficacy of drugs.
To minimize contact of airborne contamination with critical sites, facilities used to prepare ophthalmic preparations must operate in a controlled environment with HEPA filtration, as well as following cGMP guidelines. Strict adherence to aseptic technique and correct sterilization processes is vital in the preparation of ophthalmic products.
During production of eye preparations, contamination management with engineering control as an approach to achieving sterility of the product based on Revised Annex 1 Manufacture of Sterile Medicinal Product is highly urged. Systematic approaches in designing and controlling are required to minimize the possibility of sterile preparation being exposed to airborne contaminants. An ISO Class 5 and unidirectional airflow are required during evolving operating conditions, and they must be constructed to avoid contamination.
Esco Healthcare offers specialized services, equipment, and process solutions through its core platform products, supported by various core technologies. With a diverse range of innovative and ready-to-use solutions, Esco Healthcare helps various industries, including pharmaceuticals, nutraceuticals, and cosmeceuticals, meet internationally recognized GMP, environmental, and health and safety standards.
Sign up to our newsletter and receive the latest news and updates about our products!
