

Discovery to Delivery
› Solutions ›Primary Packaging
Ampoule - A container sealed by fusion and to be opened exclusively by breaking. The contents are intended for use on one occasion only.

Important Characteristics of Glass as an Adequate Material for Drugs
|
Characteristics |
Importance |
|
They can filter specific wave lengths |
Packaging of photosensitive substances |
|
High fusion point |
It tolerates the sterilization process (by vapor at 1,210°C, and dry at 2,600°C) – microbiologic control. |
|
Excellent chemical resistance |
It hardly interacts with its content |
|
Impermeability |
Important barrier between media |
|
Smooth surface |
Easy to clean |
|
Rigidity and stability |
Withstands vacuum |
|
It follows a structural pattern of a mold |
Ease to make several containers |
Pharmaceutical Ampoules
This machine is mainly used in the pharmaceutical industry for small volume ampoule cleaning.
Mainly used on the injection linked production line of pharmaceutical factories. It can continuously dry, sterilize and remove pyrogen of the washed ampoule.
The rotating gripper/transport system accepts the container smoothly and transfers to the different cleaning stations in continuous motion, where the containers are treated for pre-determined period of time.
The first cleaning station is operated with purified water, which can be recirculated. The same water quality is applied for filling the containers in front of the ultrasonic bath and the needles of the first cleaning station. By that the fresh water consumption is reduced to a minimum. A senor in the purified water tank controls the water level. A permanent renewal of the water is achieved by collecting the overflow from the final rinse station.
All water going into the needles is filtered with particle filters, which are placed in the water feed system. An additional wire cloth filter positioned in the drain of the tank in front of the recirculation pumps hold back macroscopic particles such as glass splinters, which could damage the pumps.
It performs a final rinse of the inside of the containers in order to remove particles which were detached from the glass surface durin prior steps. This step is more dilution than mechanical removal.
To avoid residues from drying of water droplets on top of the glass surface, residual water is removed with pressurized sterile filtered air.
Performed at the end of the cleaning cycle. The goal of siliconization is a homogenous coating of the inside of the container with a silicon layer, which will be cured as an integral part of the sterilization and depyrogentaion process in the heat tunnel.


Image courtesy of BAUSCH Advanced Technology Group
Ampoule Depyrogenation
Involves the sterilization of the ampoules with hot air and constantly recirculated. The ampoules are then cooled gently before they are automatically transferred into the next production process.


Image courtesy of BAUSCH Advanced Technology Group
Ampoule Dosing
The peristaltic pump uses a rotor in which multiple rollers are mounted. Partially surrounding the rotor is a stationary, curved shoe or “anvil”. A flexible, hollow tube (usually silicone rubber) is pinched between each roller and the shoe. As the rotor rotates, fluid inside the tube is driven forward as the pinched portion of the tubing advances.
The piston pump, along with the rolling diaphragm pump, may be compared to a syringe in that they both employ a moving piston inside a stationary cylinder to displace a precise amount of liquid. As the piston moves upward, liquid is forced out of the pump and when it moves downward, liquid is drawn into the pump.
The rolling diaphragm pump uses a flexible membrane (diaphragm) attached to the pump body at its outside diameter and to the piston at its inside diameter. A space between the piston and the body internal body cylinder allows the diaphragm to be “doubled” and to “roll” as the piston moves up and down. Vacuum is applied to a port in the lower portion of the pump body to maintain the shape of the diaphragm and to pull the piston downward on the refill portion of the filling cycle. Typically, the product supply has a slight overpressure.
Ampoule Filling
A time pressure filling system in its simplest form, includes a product supply vessel under controlled pressure, a valve to open and close the product flow from the tank to the filling needle, and a clock or timing device to repeatedly control the amount of time that the valve is open.
The product supply tank may or may not be pressurized, and a flow orifice may be used to improve repeatability.
The product supply tank may be pressurized at a control pressure, or may use a precisely controlled liquid level (gravity feed). For small fills, common in pharmaceutical filling, a pressurized system provides more precise fill volume control and acceptable cycle rates.
A gravimetric filling system is simply a system that uses gravity, or a controlled liquid height, to provide a consistent pressure at the metering device near the filling needle. A simple time pressure filling system or a fill by weight system may be gravimetric. These system use a “clock” and a valve (time pressure) and a scale and a valve (fill by weight) for metering doses.


Images courtesy of BAUSCH Advanced Technology Group
Ampoule Labelling
Containers are fed from an upstream machine then gently transferred to the labeling station and applied to the containers by smoothing belts and rollers.


Images courtesy of BAUSCH Advanced Technology Group
Ampoule Tray Loading
Products are collated onto tray banks, hence product stabilization is established and controlled throughout the process.


Images courtesy of BAUSCH Advanced Technology Group
Pharmaceutical Distribution Bags – a container consisting of surfaces, whether or not with a flat bottom made of flexible material, closed at the bottom and at the sides by sealing; the top may be closed by fusion of the material, depending on the intended use.
This may be used for the identification, validation, authentication, and tracking and tracing of pharmaceuticals in the supply chain, including individual units or batched of pharmaceuticals being filled, stored, packaged and transported.
Intravenous Bag Dosing
The peristaltic pump uses a rotor in which multiple rollers are mounted. Partially surrounding the rotor is a stationary, curved shoe or “anvil”. A flexible, hollow tube (usually silicone rubber) is pinched between each roller and the shoe. As the rotor rotates, fluid inside the tube is driven forward as the pinched portion of the tubing advances.
The piston pump, along with the rolling diaphragm pump, may be compared to a syringe in that they both employ a moving piston inside a stationary cylinder to displace a precise amount of liquid. As the piston moves upward, liquid is forced out of the pump and when it moves downward, liquid is drawn into the pump.
The rolling diaphragm pump uses a flexible membrane (diaphragm) attached to the pump body at its outside diameter and to the piston at its inside diameter. A space between the piston and the body internal body cylinder allows the diaphragm to be “doubled” and to “roll” as the piston moves up and down. Vacuum is applied to a port in the lower portion of the pump body to maintain the shape of the diaphragm and to pull the piston downward on the refill portion of the filling cycle. Typically, the product supply has a slight overpressure.
Intravenous Bag Filling
A time pressure filling system in its simplest form, includes a product supply vessel under controlled pressure, a valve to open and close the product flow from the tank to the filling needle, and a clock or timing device to repeatedly control the amount of time that the valve is open.
The product supply tank may or may not be pressurized, and a flow orifice may be used to improve repeatability.
The product supply tank may be pressurized at a control pressure, or may use a precisely controlled liquid level (gravity feed). For small fills, common in pharmaceutical filling, a pressurized system provides more precise fill volume control and acceptable cycle rates.
A gravimetric filling system is simply a system that uses gravity, or a controlled liquid height, to provide a consistent pressure at the metering device near the filling needle. A simple time pressure filling system or a fill by weight system may be gravimetric. These system use a “clock” and a valve (time pressure) and a scale and a valve (fill by weight) for metering doses.
Blister – a multi-dose container consisting of two layers, of which one is shaped to contain the individual doses. Strips are excluded.
Uses of Blister Packaging in Pharmaceutical Industry
Advantages of Blister Type Packaging
Blister Packaging Machinery
Modern thermoform-fill-seal machined can operate at speeds ≤ 800 packages/min. Emphasis in improving production is placed on applying microprocessor controls that electronically connect the filling and forming equipment with other downstream machinery for cartoning and wrapping. These controls also feed tablets or liquids into the unit-dose blisters, ensuring that an exact volume is put into each. Modern machinery also uses integrated vision systems to help ensure the accuracy of the fill and the integrity of the product in the blister. These machines have become quite versatile and can readily accommodate several lidstocks and basestock, allowing the manufacturer to obtain better compatibility between the medicine and its packaging material as well as better patient compliance.
Bottle – a container with a more or less pronounced neck and usually a flat bottom.
Cartridge – usually cylindrical, suitable for liquid or solid pharmaceutical dosage forms; generally for use in a specifically designed apparatus. (e.g. prefilled syringes).

Cartridge Cleaning
Cartridge Depyrogenation
Involves the sterilization of the cartridge with hot air and constantly recirculated. The cartridge are then cooled gently before they are automatically transferred into the next production process.
Cartridge Dosing
The peristaltic pump uses a rotor in which multiple rollers are mounted. Partially surrounding the rotor is a stationary, curved shoe or “anvil”. A flexible, hollow tube (usually silicone rubber) is pinched between each roller and the shoe. As the rotor rotates, fluid inside the tube is driven forward as the pinched portion of the tubing advances.
The piston pump, along with the rolling diaphragm pump, may be compared to a syringe in that they both employ a moving piston inside a stationary cylinder to displace a precise amount of liquid. As the piston moves upward, liquid is forced out of the pump and when it moves downward, liquid is drawn into the pump.
The rolling diaphragm pump uses a flexible membrane (diaphragm) attached to the pump body at its outside diameter and to the piston at its inside diameter. A space between the piston and the body internal body cylinder allows the diaphragm to be “doubled” and to “roll” as the piston moves up and down. Vacuum is applied to a port in the lower portion of the pump body to maintain the shape of the diaphragm and to pull the piston downward on the refill portion of the filling cycle. Typically, the product supply has a slight overpressure.
Cartridge Filling
A time pressure filling system in its simplest form, includes a product supply vessel under controlled pressure, a valve to open and close the product flow from the tank to the filling needle, and a clock or timing device to repeatedly control the amount of time that the valve is open.
The product supply tank may or may not be pressurized, and a flow orifice may be used to improve repeatability.
The product supply tank may be pressurized at a control pressure, or may use a precisely controlled liquid level (gravity feed). For small fills, common in pharmaceutical filling, a pressurized system provides more precise fill volume control and acceptable cycle rates.
A gravimetric filling system is simply a system that uses gravity, or a controlled liquid height, to provide a consistent pressure at the metering device near the filling needle. A simple time pressure filling system or a fill by weight system may be gravimetric. These system use a “clock” and a valve (time pressure) and a scale and a valve (fill by weight) for metering doses.
Cartridge Labelling
Containers are fed from an upstream machine then gently transferred to the labeling station and applied to the containers by smoothing belts and rollers.
Cartridge Tray Loading
Products are collated onto tray banks, hence product stabilization is established and controlled throughout the process.
Gas Cylinder – usually cylindrical, suitable for compressed, liquefied or dissolved gas, fitted with a device to regulate the spontaneous outflow of gas at atmospheric pressure and room temperature.
General case: cylinders of single gases
Validation of the cylinder filling process is performed by a weighing (or double weighing) control, including the calculation of the mean, standard deviation and coefficients of variation, or by pressure if justified.
This validation should ideally be presented for all types of cylinder, but at least for critical types of containers. A bracketing approach may be used as a function of the capacity, material used for construction and whether fitted with a built-in pressure regulator or a residual pressure valve. If needs be, for each type of alloy used, a single validation can be performed on one cylinder of this alloy, on the condition that this is justified.
Validation can be performed by determining the amount of gas contained in a cylinder compared with a reference cylinder filled with the charge of gas to avoid the problems of fluctuations in pressure as a function of temperature. The reproducibility of filling is also verified whatever the composition of the finished product batch (homogenous or heterogeneous).
For compressed gases, the temperature and pressure stabilisation time after filling which depends upon various capacities, nature of material, thermal exchange, ambient temperature (and any variation in it during the stabilisation time), rate of filling of the cylinder, airflow over the cylinder and its proximity to any adjacent filled cylinders is specified, unless otherwise justified. Cylinders, which have been returned for filling, are prepared in accordance with Annex 6 of the GMP.
The integrity of the filling system to indicate leak tightness to prevent possible contamination of the system under vacuum or an estimate of the yield accounted for losses as an annual average is provided. The tolerated limits of temperature and pressure are provided (specifying especially the hydrostatic pressure test and the bursting pressure of the cylinder). On the safety level, any problems of possible overload of compressed or liquefied gas are addressed.
Other containers for single gases
In the case of cylinder bundles and mobile evaporators, validation of the filling procedure is also performed by weighing or by pressure if justified.
In the case of fixed evaporators, validation can consist of the absence of impurity enrichment due to the formation of degradation products with time, to trapping because of the temperature and to transfers from one container to another during the manufacturing process or during sampling. It is specified whether the impurities remain at the same proportions between the gaseous and liquid phases as the container is emptied. The minimal threshold for filling the fixed evaporator is specified to avoid any risk of impurity enrichment.
Analytical Procedure
In the case of liquefied gases, the nature of the phase (liquid or gaseous) is indicated, as is the method of sampling for the control. In the case of impurities that are preferentially present in the gaseous phase and eliminated to a large extent during the first drawing off (e.g. nitric oxide in medical nitrous oxide), the order of analyses is specified.
Medicinal gases are often packaged in a wide range of containers:
− compressed gas cylinders,
− liquefied gas cylinders, with or without a dip tube,
− cylinder bundle,
− mobile evaporator,
− fixed evaporator,
− mobile cryogenic container,
− fixed cryogenic container.
A variety of reference codes exist, depending on the supplier, capacity and material, particularly in the case of cylinders. For each reference and each capacity, the water capacity of the container (in L), the amount of gaseous product released at 1atm and 15°C (in m3), and the weight of product stored (in g for compressed gases or in kg for liquefied gases) are provided, together with the accepted deviations. For liquefied gas cylinders, the pressure remains constant then falls suddenly at the end of use. Therefore, only the weight monitors the state of filling. The filling pressure (at 15° C) is justified in comparison with the weight formula.
Injection needle – a hollow needle with a locking device intended for the administration of liquid pharmaceutical dosage forms.
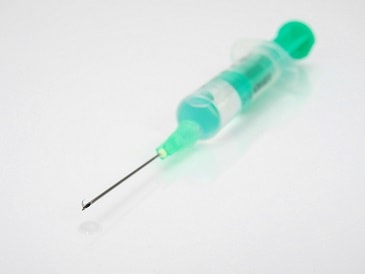
Injection syringe – a cylindrical device with a cannula-like nozzle, with or without a fixed needle and a movable piston, used for the administration, usually parenteral, of an accurately measured quantity of a liquid pharmaceutical form. The syringe may be prefilled, and can be for single-dose or multi-dose use.

Types of Injection Syringe
Pressurized container – suitable for compressed, liquefied or dissolved gas fitted with a device that, after its actuation, produces a controlled spontaneous release of the contents at atmospheric pressure and room temperature.
Aerosol Filling Methods
This method has a fill accuracy typically + or – ½ mL and capable of filling many different product types and viscosities up to 50,000 centipoise.
Advantages:
Disadvantages
Advantages
Disadvantages
Nitrous oxide and carbon dioxide are used as propellants to deliver food products.
Method 1 – Gas is mixed with the product in saturation towers until blended properly. The product and CO2 are filled into the aerosol container as one.
Method 2 – Gas is injected through the aerosol valve stem using time over high pressure. Sometime the CO2 is heated to aid in saturation. Shaking is common during gassing.
Method 3 - CO2 is injected under the aerosol valve cup using time over pressure. Restricted valve lift helps to create turbulence for saturation.
Advantages:
Disadvantages
Single-dose container – a container consisting of two layers, usually provided with perforations, suitable for containing single doses of solid or semi-solid preparations. Blisters are excluded.
Tube – a container for multi-dose semi-solid pharmaceutical forms consisting of collapsible material; the contents are released via a nozzle by squeezing the package.
Filling of semi-solid dosage forms
Vial – A small container for parenteral medicinal products, with a stopper and over seal; the contents are removed after piercing the stopper.

Vial Cleaning
Vial Depyrogenation
Involves the sterilization of the vial with hot air and constantly recirculated. The ampoules are then cooled gently before they are automatically transferred into the next production process.
Vial Dosing
The peristaltic pump uses a rotor in which multiple rollers are mounted. Partially surrounding the rotor is a stationary, curved shoe or “anvil”. A flexible, hollow tube (usually silicone rubber) is pinched between each roller and the shoe. As the rotor rotates, fluid inside the tube is driven forward as the pinched portion of the tubing advances.
The piston pump, along with the rolling diaphragm pump, may be compared to a syringe in that they both employ a moving piston inside a stationary cylinder to displace a precise amount of liquid. As the piston moves upward, liquid is forced out of the pump and when it moves downward, liquid is drawn into the pump.
The rolling diaphragm pump uses a flexible membrane (diaphragm) attached to the pump body at its outside diameter and to the piston at its inside diameter. A space between the piston and the body internal body cylinder allows the diaphragm to be “doubled” and to “roll” as the piston moves up and down. Vacuum is applied to a port in the lower portion of the pump body to maintain the shape of the diaphragm and to pull the piston downward on the refill portion of the filling cycle. Typically, the product supply has a slight overpressure.
Vial Filling
A time pressure filling system in its simplest form, includes a product supply vessel under controlled pressure, a valve to open and close the product flow from the tank to the filling needle, and a clock or timing device to repeatedly control the amount of time that the valve is open.
The product supply tank may or may not be pressurized, and a flow orifice may be used to improve repeatability.
The product supply tank may be pressurized at a control pressure, or may use a precisely controlled liquid level (gravity feed). For small fills, common in pharmaceutical filling, a pressurized system provides more precise fill volume control and acceptable cycle rates.
A gravimetric filling system is simply a system that uses gravity, or a controlled liquid height, to provide a consistent pressure at the metering device near the filling needle. A simple time pressure filling system or a fill by weight system may be gravimetric. These system use a “clock” and a valve (time pressure) and a scale and a valve (fill by weight) for metering doses.
Vial Sealing
Vials and bottle are sealed by closing the opening with a rubber closure or stopper.
When rubber closures are to be inserted mechanically, the surface of the closure is often “halogenated” or “siliconized” to give it less friction.
Mechanical stoppering has been developed to meet the need for high speed production.
Rubber closures are held in place by means of aluminum caps.
Vial Labelling
Containers are fed from an upstream machine then gently transferred to the labeling station and applied to the containers by smoothing belts and rollers.
Vial Tray Loading
Products are collated onto tray banks, hence product stabilization is established and controlled throughout the process
Sign up to our newsletter and receive the latest news and updates about our products!
