Upstream process is the first step of bioprocess from early cell isolation and cultivation, to cell banking and culture development of the cells until final harvest where the desired quantity is reached. Since this is the early stage of bioprocessing, the quality of the product is of critical importance. Sustainability of this procedure must also be considered. Thus, compliance to cGMP standards is required. One of the measurable requirement is to warrant that from this stage to the filling and packaging must ensure sterility of the process. These parameters will include the use of equipment like bioreactors and cell therapy aseptic isolators, presence of a clean room, and only certified biotechnologist and technicians will be allowed to handle the delicate living organisms.
Cell isolation is one of the most basic practices in cell culture studies and there are various techniques to do this. The importance of these two processes lie in the goal of understanding certain cell mechanisms in order to formulate new biological drugs and combat specific disease types. To do this, a detailed biochemical analysis is required. Most procedures require a large number of cells that must be physically disrupted so their components can be further isolated and proliferated in pure culture for further analysis.
If the target cell is part of a tissue, it must first be dissociated and separated in various types. Disrupting the extracellular matrix holding the cells together is the first step to isolate specific cells from a tissue sample; and as studies suggest, the best source of viable dissociated cells are those from fetal or neonatal tissues.
The process of disrupting the matrix involves treating the tissue sample with proteolytic enzymes and chelating agents: the former will digest the proteins in the extracellular matrix, while the latter will be binding or chelating the calcium (Ca2+) in the matrix to break cell-cell adhesion. Afterwards, proceed with gentle agitation to attain available single living cells. However, the sample achieved from this process is composed of several types of cells, so the next part involves the isolating the different cell types from the suspension.
Isolating cells of different types from a suspension involves taking advantage of their variation in physiology. This is better exploited by centrifugation and the capacity of other cells to strongly adhere to glass or plastic and, a much refined technique, to a specific antibody that can only bind to one cell type.
However, an even more sophisticated method for cell separation is through the use of an antibody combined to a fluorescent dye. This combination is used to label specific cells so they can be easily separated by non-labelled ones via an electronic fluorescence-activated cell sorter. This machine can easily sort out 1 fluorescent cell from a pool of 1000 unlabelled once and can sort cells a thousandth per second! It does this by allowing individual cells to travel in a single file through a laser beam where the fluorescence of each cell is measured rapidly.
Another form of cell isolation is the laser capture microdissection (LCM) which allows isolation of pure cells from a heterogenous mix or from live cells and even cytological preparations. DNA, RNA, and protein biomolecules extracted by this process can be used for downstream applications such as polymerase chain reaction expression studies, and microarray and proteomic analysis.
As mentioned earlier, most procedures require a large number of cells that must be physically disrupted so their components can be further isolated and proliferated in pure culture for further analysis.
Once specific cells have been isolated from the source, they can then be cultured to allow a much detailed biochemical analysis as well as other formulation studies to be done. More applications of cell culture are for the fields of: monoclonal antibodies, vaccines, enzymes, hormones, interleukins, and certain growth factors.
Cell culture basically refers to growing isolated cells in an artificial environment which mimics the conditions of their natural one ~ complete with nutrients as well as certain temperature settings.
Different Types of Cell Cultures:
- Primary Culture: This is a preparation of cells directly from a parent plant or animal tissue via mechanical or enzymatic measures. Cell proliferation is then maintained through a suitable media in a glass or plastic container whose conditions mimic the original environment. This process can be done without the need to separate different cell types as discussed previously.
Cells in a primary culture all have the same karyotype similar to their tissue of origin. Karyotype refers to the same number and appearance of chromosomes in the nucleus of a (eukaryotic) cell.
It must be noted that primary cultured cells will have a limited lifespan because of the substrate and nutrient exhaustion which can cause toxin build up and dampen further cell growth.
There are two different types of primary cell culture based on the kind of cells used:
-
Adherent Cells: refers to cells requiring a matrix attachment for growth. These are normally isolated from organ tissues where the cells are immobile and surrounded of connective tissues.
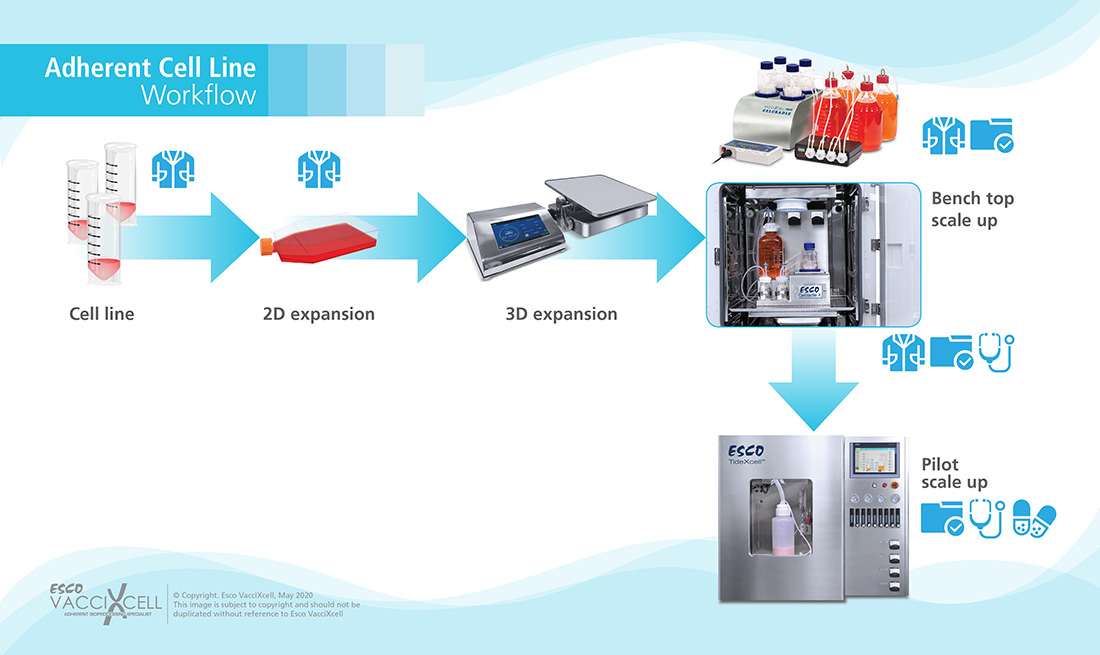 Figure 1. Adherent Cell Workflow
Figure 1. Adherent Cell Workflow
-
Suspension Cells: opposite to adherent ones, these do not need anchorage or attachment to grow. Such cells are taken from the blood system.
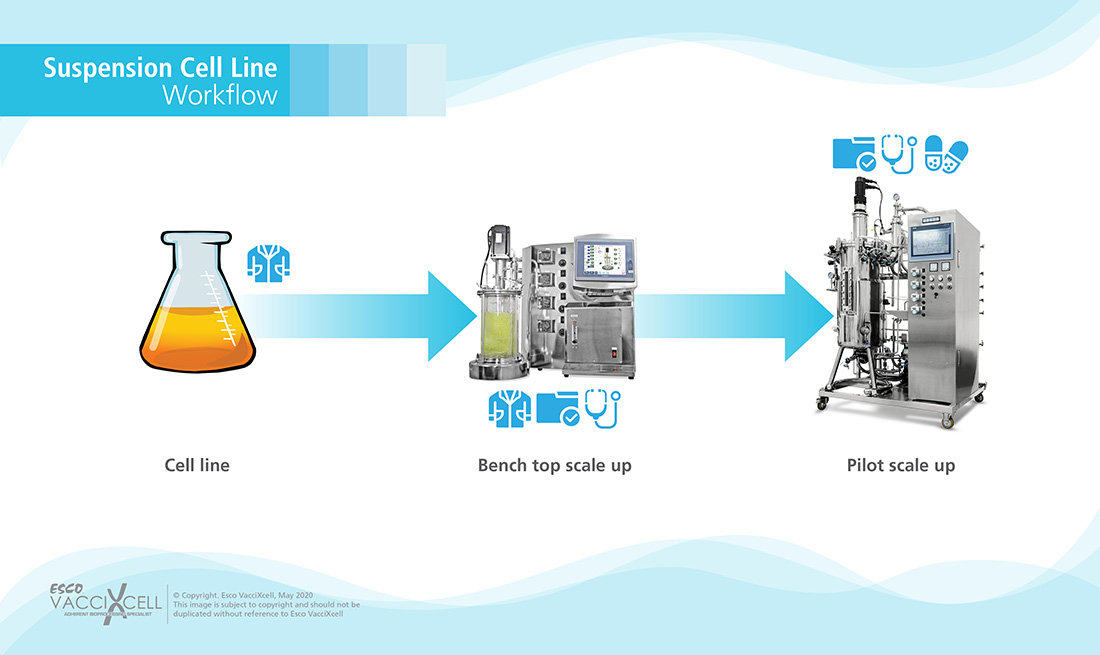 Figure 2. Suspension Cell Workflow
Figure 2. Suspension Cell Workflow
- Secondary Culture: During this process, the cells from the primary culture are transferred to a new vessel equipped with fresh media to facilitate continuous cell proliferation. This process involves disassociating the adhered cells in adherent primary cultures. The importance of this secondary culture is that normally, the amount of cells derived from primary cell culture is insufficient for detailed researches, therefore, with secondary culture, cell population is increased along with their lifespan.
This process is applicable for generating replicated cultures for characterization, preservation, and further experimentation.
This has become a normal procedure for lots of scientists working with primary and/or secondary cell cultures or even cell lines. However, they all encounter the same problems such as:
- Slow growth
- Spontaneous differentiation
- Evaporation
- Contamination, etc.
References:
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter P. (2002). Molecular Biology of the Cell: Isolating Cells and Growing Them in Culture (4th ed.). National Center for Biotechnology Information. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK26851/
- Centers for Disease Control and Prevention. (2018). Understanding How Vaccines Work. Retrieved from: https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf
- Pedraza, J. (2016). What is the difference between primary and secondary cell culture?. ResearchGate. Retrieved from: https://www.researchgate.net/post/What_is_the_difference_between_primary_and_secondary_cell_culture#:~:text=Primary%20cell%20culture%20is%20the,containers%20under%20controlled%20environmental%20conditions
- ScienceDirect. (n.d.). Cell Isolation. Retrieved from: https://www.sciencedirect.com/topics/immunology-and-microbiology/cell-isolation
- ScienceDirect. (n.d.). Cell Lines. Retrieved from: https://www.sciencedirect.com/topics/neuroscience/cell-lines
- ScienceDirect. (n.d.). Laser Capture Microdissection. Retrieved from: https://www.sciencedirect.com/topics/neuroscience/laser-capture-microdissection
- STEMCELL Technologies. (n.d.). Cell Separation and Cell Isolation Methods. Retrieved from: https://www.stemcell.com/cell-separation/methods#section-immunomagnetic
- Uysal, O., Sevimli, T, Sevimli, M., Gunes, S., & Sariboyaci, A. (2018). Cell and Tissue Culture: The Base of Biotechnology. ScienceDirect. doi: https://doi.org/10.1016/B978-0-12-804659-3.00017-8
- Xu, C., Hu, S., & Chen, X. (2016). Artificial cells: from basic science to applications. materialstoday, 19(9), 516-532. doi: https://doi.org/10.1016/j.mattod.2016.02.020
Recommended Products
Cell proliferation refers to the increase in number of cells as a direct result of normal cell growth and division. During the process of vaccine/biological drug formulations, specific cells are targeted and isolated for their benefits to boost patient’s health. Once the target cell has been isolated, it is now time to grow them artificially in the laboratory in order to create biological drugs.
Regulating Cell Proliferation
Cell proliferation is regulated by the presence of several factors such as nutrient levels, temperature, pH, and oxygen, among others, in the internal environment. Cells also use special proteins and specific signalling systems to ensure its proper cycle progression.
Before a cell in the resting phase [Gap 0 (G0)] can enter the cell cycle process (G1), it must first be stimulated by a specific protein commonly referred to as the growth factor. Such proteins responsible for activating G0 cells are categorized into four major groups: growth factors, growth factor receptors, signal transducers, and nuclear regulatory proteins. Cell growth is activated via direct stimulation of the cell’s nucleus. To do this, a growth factor must bind to its receptor located on the cell membrane, which will then be temporarily activated. Afterwards, a signal will be transmitted from the cell surface receptor towards the nucleus; therefore, triggering gene transcription involved in cell proliferation.
Normally, cell proliferation is composed of four major stages:
- Interphase: Gap 1 (G1) phase
- This is the phase where the cell increases in size and is preparing to divide.
- Interphase: Synthesis (S) phase
- The phase where cellular DNA is copied.
- Interphase: Gap 2 (G2) phase
- Copies of cellular DNA will then be organized and condensed; cell prepares to divide.
- Mitosis (M) phase
- Cell division process wherein two copies of the genetic material is partitioned into two daughter cells.
As mentioned earlier, cells also use a signalling system to guarantee proper cell proliferation.
- End of G1 and beginning of G2 checkpoint: helps assess any possible damage in the DNA before and after synthesis.
- Mitosis checkpoint: ensures that all the spindle fibers are properly arranged (metaphase) prior to chromosomal separation (anaphase).
Normally, any abnormalities detected during such events will force apoptosis or programmed cell death. But in the presence of defective proteins, no apoptosis is triggered, rather, malignant transformation can occur which can later on lead to cancer.
In the field of biopharmaceuticals, one way to ensure a reproducible and standardized method of cell proliferation is through the use of bioreactors. Such equipment systems are indispensable tools for cell-based therapies as they maintain a well-controlled microenvironment wherein cells can easily grow.
Bioreactors have been extensively used for the expansion of red blood cells, chimeric antigen receptor (CAR) T cells, induced pluripotent stem cells, and even mesenchymal stem cells.
There are many different types of bioreactors available in the market today for adherent culture. Choosing the right bioreactor system for cells is crucial for cell culture success. The choice of the bioreactor system to use highly depends on the type of cells to be produced, the desired product, the process of cell culture, and the product density.
As part of Esco Healthcare’s division, Esco VacciXcell offers different types of bioreactors for different types of adherent cells.
 Figure 3. Tide Technology for bioreactors
Figure 3. Tide Technology for bioreactors
References:
- Anderson, H, (n.d.). Cell Proliferation and Cell Cycle: Definitions Vs Differentiation. Microscope Master. Retrieved from: https://www.microscopemaster.com/cell-proliferation.html
- Brody, L. (n.d.). Cell Cycle. National Human Genome Research Institute. Retrieved from: https://www.genome.gov/genetics-glossary/Cell-Cycle
- Nature Research. (n.d.). Cell Proliferation. Retrieved from: https://www.nature.com/subjects/cell-proliferation#:~:text=Cell%20proliferation%20is%20the%20process,through%20cell%20death%20or%20differentiation.
- ScienceDirect. (n.d.). Cell Proliferation. Retrieved from: https://www.sciencedirect.com/topics/medicine-and-dentistry/cell-proliferation
- Stephenson, M., & Grayson, W. (2018). Recent advances in bioreactors for cell-based therapies. National Center for Biotechnology Information. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5931275/#:~:text=Bioreactors%20for%20cell%20proliferation%20and%20differentiation,-The%20therapeutic%20promise&text=A%20few%20types%20of%20bioreactors,cellular%20responses%20to%20microenvironmental%20cues.
- The Editors of Encyclopaedia Britannica. (2020). Vacuole. Britannica. Retrieved from: https://www.britannica.com/science/vacuole
- Yang, N., Ray, S.D., & Krafts, K. (2014). Cell Proliferation. ScienceDirect. Retrieved from: https://www.sciencedirect.com/science/article/pii/B9780123864543002748
Recommended Products
Cell banking refers to the collection, processing, and storage of specific cell lines, especially stem cells, with the capacity to provide life-saving biopharmaceutical products. Cell banking offers a reproducible way to manufacture high-quality, safe, and efficient biological products that have undergone proper characterization. Processes involved in cell banking are safe, non-invasive and risk free.
Specific cell lines can be stored in cell banks via cryopreservation process which can last for over 25 years. Cryopreservation utilizes very low temperatures proven to reduce biological and chemical reaction rates in order to preserve cells, organelles, tissues, and/or any other biologicals. However, this process risks of fatally injuring the cells due to the formation of ice crystals and even osmotic changes, leading to cellular mechanical constraints. To address such issue, a cryoprotective agent (CPA) is used which affects ice crystal growth, nucleation, and even the rate of water transport.
Cryopreservation places biological samples in a state of suspended animation to preserve the fine structure of cells within any time span, and such cryopreserved cells, or even tissues, possess certain advantages for researches and even future clinical studies.
Moreover, this constant availability of cryopreserved cells and tissues allow for an exhaustive quality testing to determine transplantation suitability without the need of obtaining fresh samples.
Cryopresevation Methods
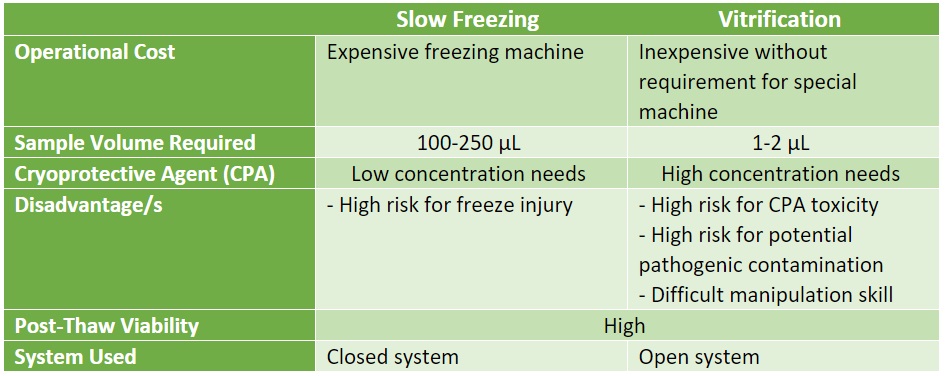 Table 1. Different methods of Cryopreservation
Table 1. Different methods of Cryopreservation
- Slow Freezing: This process allows the substitution of cytoplastic water with CPAs in order to reduce cell damage and to adjust cooling rate relevant to the cell membrane’s permeability. Theoretically, slow cooling can cause cells to rapidly leak intracellular fluids so it can eliminate super-cooling and ice formation; and since each cell have different permeability capabilities, optimal cooling rate is dependent on cell type
Protocols for slow freezing involve an estimated cooling rate of 1°C/min with < 1.0M CPA with a high-cost controlled rate freezer or a benchtop portable freezing container.
Advantages:
- Low risk of contamination
- No need for manipulation skills
Disadvantage/s:
- High risk of cellular injury due to extracellular ice formation.
-
Vitrification: This is also known as ‘ice-free’ cryopreservation utilizing high concentrations of CPAs in order to completely prevent ice formation. CPAs used in this process are at concentrations of 4 to 8 M. However, since each cell varies in their sensitivity or toxicity response to such high concentrations of CPA, there must be different protocols optimized for each.
Vitrification is dependent on three (3) factors:
- Sample viscosity
- Cooling and warming rates
- Sample volumes
Methods of Vitrification:
- Equilibrium: This process requires the formulation of multimolar mixtures of cryoprotective agents (CPAs) and their injection into the cell suspensions.
- Nonequilibrium: Vitrification using this method makes use of either a carrier-based (quartz microcapillaries and cryoloops, among others) or carrier-free system in an extremely high freezing rate condition with lower CPA mixture concentration.
Cell banking protocols must be properly optimized for each cell type in order to develop and maintain cell viability, recovery, potency, and overall, to guarantee final biopharmaceutical product safety.
References:
- Akel, S.(2015). Cell Banking: Process Development and Cell Preservation. ScienceDirect. Retrieved from: https://www.sciencedirect.com/science/article/pii/B9780124103962000037
- Charles River. (n.d.). Cell Banking. Retrieved from: https://www.criver.com/products-services/biologics-testing-solutions/manufacturing-production/cell-banking?region=3701
- Future Health Biobank. (n.d.). What is stem cell banking. Retrieved from: https://futurehealthbiobank.com/
- Hanna, J., & Hubel, A. (2009). Preservation of stem cells. Organogenesis, 5(3), 134-137. doi: 10.4161/org.5.3.9585
- Jang, T., Park, S., Yang, J., Kim, J., Seok, J., Park, U., Choi, C., Lee, S., & Han, J. (2017). Cryopreservation and its clinical applications. Integrative Medicine Research, 6(1), 12-18. doi: 10.1016/j.imr.2016.12.001
As part of the biopharmaceutical manufacturing industry, it is important to ensure the timely availability and reproducibility of all raw materials and key ingredients. The process of fermentation is critical in the said industry as it promotes the formation of biopharmaceuticals, food/feed supplements, biofuels, and even chemical building blocks.
Since it is a critical process, bioprocess scientists and engineers must optimize a reproducible and cost-effective way to sustain it. To do so the following factors must be considered:
- Costs for media and supplements
- Process runtime
- Bacterial growth and viability
- Product titer and yield
- Product quality
- Culture medium concentrations of nutrients and by-products
Process development is also the same time that bioprocess engineers will determine the best fermentation approach for the specific cells or samples they would be using.
Fermentation Methods
- Batch Fermentation: This approach uses a fixed volume of medium inside the fermentor wherein the microorganisms will be inoculated. The culture medium needs to be changed periodically to prevent the saturation of the medium with the cells and their by-products.
There are different phases of growth that the microorganisms will be subjected to: the initial lag, the exponential growth, the stationary growth, and the death phase.
Initial lag phase refers to the period wherein the cells are accustoming to the new environment presented by the fermentor and the culture media; therefore, growth here is slow. Although, once the cells fully adapt to the conditions of the media they will now be entering the exponential phase or log phase wherein cell division occurs. Predictably, this phase is marked with a doubling of cell population. As long as the environmental conditions of the cells are optimal, rapid cell growth will be seen and will be marked with a steep slope on the growth curve.
However, once the increased cell population uses up the essential nutrients present in the culture media, they will now be entering the stationary growth phase. By this time, the number of new cells produced is equal to the number of cells dying off, causing a flattening on the growth curve. Notably, new cells produced in this phase try to adapt to the starvation conditions, thus, they are smaller in size, their plasma membrane less fluid and less permeable, and their nucleoid condenses with their DNA becoming bound with DNA-binding proteins from starved cells (DPS). Moreover, cells produced in the stationary phase are prone to yield secondary metabolites which can be antibiotics. Any other cells capable of the sporulation process will be activated at this point in time.
The last phase is the death or decline phase wherein the number of viable cells decrease in an exponential way. By this time the culture conditions may have already deteriorated to the point of injuring or damaging the cells in an irreparable way. Notably, studies suggest that 100% cell death is highly unlikely as cells mutate and adapt even in harsh conditions. Thus, a tailing effect is observed wherein a small cell population cannot be killed off and are getting their nutrients from the lysis of dead cells in the same culture.
Studies show of a condition called viable but nonculturable (VBNC) which suggests that dead cells can be revived. This research may be of prime importance to pathogens which are capable of entering a state of low metabolism and no cell division in harsh conditions, but resume such activities with better culture conditions.
Advantages of Batch Processing:
- Ease of operation
- Low contamination risk
Disadvantages of Batch Processing:
- Low cell densities
- Long downtime between batches caused by cleaning, vessel setup, and sterilization
For initial studies and establishment of experimental design, batch fermentation offers a convenient method to optimize culture conditions.
- Fed-batch Fermentation: This is a widely used process in the bioprocess industry and is a modified version of the batch fermentation method. In fed-batch fermentation the microorganisms are inoculated and grown while nutrients are added in increments into the fermenter. This process lasts throughout the entire duration of the fermentation to feed the microorganisms. Designing the timeframe for feeding should not allow an excessive saturation of the substrate in order to properly support inoculate growth; especially since the period for feeding is usually marked by a substrate limitation in the broth. At the end of each run, the culture suspension is removed.
Fed-batch fermentation process is useful for bioprocesses requiring high product yield.
Advantages of Fed-batch Fermentation:
- Essential for bioprocesses wherein substrate inhibition is required.
- Fed-batch can help control the rate at which the substrate is fed so maximum yield of the desired product is attained.
- Efficient way of accumulating non-growth associated products (e.g. antibiotics)
- Overcoming phenomenon of catabolite repression
- Possible to use organic solvents as nutrient-source
- Possible to control the reaction rate and metabolic reaction via manipulation of the feeding of the limiting substrate.
-
Continuous Fermentation: This process provides fresh medium and harvests cultured cells while removing used medium all at the same time. The constant removal of toxic wastes and used up medium allows the culture volume to stay constant; unlike with fed-batch fermentation, the maximum volume of the vessel does not influence the amount of fresh medium that can be added into the culture throughout the whole process.
Continuous fermentation is commonly used in an industrial scale.
Advantages of Continuous Fermentation:
- Simplifies culture scale-up
- Steady state can be achieved wherein rate of cellular growth and environmental conditions remain constant.
- Longer lifespan for cultured samples
- Reduces process downtime
- Cost-effective
Disadvantages of Continuous Fermentation:
- Challenging sterility maintenance due to long cultivation periods
References:
- Bruslind, L. (2020). Microbial Growth. LibreTexts. Retrieved from: https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Bruslind)/09%3A_Microbial_Growth
- Khan Academy. (n.d.). Cellular Respiration: Fermentation and anaerobic respiration. Retrieved from: https://www.khanacademy.org/science/ap-biology/cellular-energetics/cellular-respiration-ap/a/fermentation-and-anaerobic-respiration
- Lim, H., & Shin, H. (2013). Classification and Characteristics of Fed-Batch Cultures. In Fed-Batch Cultures: Principles and Applications of Semi-Batch Bioreactors (Cambridge Series in Chemical Engineering, pp. 62-84). Cambridge: Cambridge University Press. doi:10.1017/CBO9781139018777.006
- Maxon, W. (1955). Continuous Fermentation: A Discussion of Its Principles and Applications. Applied Microbiology, 3(2), 110-122. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1057071/?page=1
- Mears, L., Feldman, H., Falco, F., Bach, C., Wu, M., Norregaard, A., & Gernaey, K. (2017). Continuous Fermentation for Biopharmaceuticals?. Wiley Online Library. Retrieved from: https://onlinelibrary.wiley.com/doi/10.1002/9781119001348.ch6
- Moslamy, S. (2019). Application of Fed-Batch Fermentation Modes for Industrial Bioprocess Development of Microbial Behaviour. Annals of Biotechnology & Bioengineering. Retrieved from: http://www.remedypublications.com/open-access/application-of-fed-batch-fermentation-modes-for-industrial-bioprocess-development-of-microbial-behaviour-16.pdf
- Moulton, G. (2014). Fed-Batch Fermentation (1st ed.). Elsevier.
- ScienceDirect. (n.d.). Continuous Fermentation. Retrieved from: https://www.sciencedirect.com/topics/engineering/continuous-fermentation
- ScienceDirect. (n.d.). Exponential Phase. Retrieved from: https://www.sciencedirect.com/topics/engineering/exponential-phase#:~:text=Following%20a%20period%20during%20which,to%20the%20initial%20biomass%20concentration.
- ScienceDirect. (n.d.). Fed Batch Fermentation. Retrieved from: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/fed-batch-fermentation
- Yang, Y., & Sha, M. (n.d.). A Beginner’s Guide to Bioprocess Modes – Batch, FedBatch, and Continuous Fermentation. Eppendorf. Retrieved from: https://www.eppendorf.com/product-media/doc/en/763594/Fermentors-Bioreactors_Application-Note_408_BioBLU-f-Single-Vessel_A-Beginner%E2%80%99s-Guide-Bioprocess-Modes-Batch_Fed-Batch-Continuous-Fermentation.pdf
- Yates, G., & Smotzer, T. (2007). On the lag phase and initial decline of microbial growth curves. J Theor Biol, 244(3), 511-7. doi: 10.1016/j.jtbi.2006.08.017
Recommended Products






