

Discovery to Delivery
› Solutions ›Vaginal and Rectal
Solutions may be prepared from powders as indicated earlier or from liquid solutions or liquid concentrates. In using liquid concentrates, the patient is instructed to add the prescribed amount of concentrate (usually a teaspoonful or capful) to a certain amount of warm water (frequently a quart). The resultant solution contains the appropriate amount of chemical agents in proper strength. The agents are similar to the ones described for douche powders.
Powders are used to prepare solutions for vaginal douche, i.e., for irrigation cleansing of the vagina. The powders themselves may be prepared and packaged in bulk or as unit packages. A unit package is designed to contain the appropriate amount of powder to prepare the specifi ed volume of douche solution. The bulk powders are used by the teaspoonful or tablespoonful in preparation of the desired solution. The user simply adds the prescribed amount of powder to the appropriate volume of warm water and stirs until dissolved.
Among the components of douche powders are the following:
Douche powders are used for their hygienic effects. A few douche powders containing specific therapeutic anti-infective agents such as those mentioned in the discussion of vaginal suppositories are used against monilial and trichomonal infections.
Retention Enema
A number of solutions are administered rectally for local effects (e.g., hydrocortisone) or for systemic absorption (e.g., aminophylline). In the case of aminophylline, rectal administration minimizes the undesirable gastrointestinal reactions associated with oral therapy. Clinically effective blood levels of the agents are usually obtained within 30 minutes following rectal instillation. Corticosteroids are administered as retention enemas or continuous drip as adjunctive treatment of some patients with ulcerative colitis.
Evacuation Enema
Rectal enemas are used to cleanse the bowel. Commercially, many enemas are available in disposable plastic squeeze bottles containing a premeasured amount of enema solution. The agents are solutions of sodium phosphate and sodium biphosphate, glycerin and docusate potassium, and light mineral oil. Instruction from a pharmacist is advantageous to ensure that the patient correctly uses these products. The patient should be advised to gently insert the tip of the product with steady pressure and be told that it is not absolutely necessary to squeeze all of the contents out of the disposable plastic bottle. The patient should be told that the product will most probably work within 5 to 10 minutes.
Suppositories are solid dosage forms intended for insertion into body orifices where they melt, soften, or dissolve and exert local or systemic effects. The derivation of the word suppository is from the Latin supponere, meaning “to place under,” as derived from sub (under) and ponere (to place).
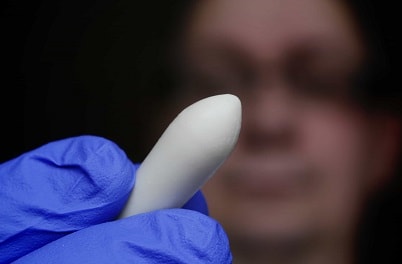
Rectal Suppositories
Rectal suppositories are usually about 32 mm (1.5 in.) long, are cylindrical, and have one or both ends tapered. Some rectal suppositories are shaped like a bullet, a torpedo, or the little fi nger. Depending on the density of the base and the medicaments in the suppository, the weight may vary. Adult rectal suppositories weigh about 2 g when cocoa butter (theobroma oil) is employed as the base. Rectal suppositories for use by infants and children are about half the weight and size of the adult suppositories and assume a more pencillike shape.
Urethral Suppositories
Suppositories for urethral administration tend to be thinner and tapered, often about 5 mm in diameter. They have been used in the treatment of local infections, and a much smaller urethral suppository has been introduced for the administration of alprostadil in the treatment of erectile dysfunction.
Vaginal Suppositories
These preparations are employed principally to combat infections in the female genitourinary tract, to restore the vaginal mucosa to its normal state, and for contraception. The usual pathogenic organisms are Trichomonas vaginalis, Candida (Monilia) albicans or other species, and Haemophilus vaginalis. Among the anti-infective agents in commercial vaginal preparations are nystatin, clotrimazole, butoconazole nitrate, terconazole, and miconazole (antifungals) and triple sulfas, sulfanilamide, povidone iodine, clindamycin phosphate, metronidazole, and oxytetracycline (antibacterials). Nonoxynol-9, a spermicide, is employed for vaginal contraception. Estrogenic substances such as dienestrol are found in vaginal preparations to restore the vaginal mucosa to its normal state.
The most commonly used base for vaginal suppositories consists of combinations of the various molecular weight polyethylene glycols. To this base is frequently added surfactants and preservative agents, commonly the parabens. Many vaginal suppositories and other types of vaginal dosage forms are buffered to an acid pH usually about 4.5, consistent with the normal vagina. This acidity discourages pathogenic organisms and provides a favorable environment for eventual recolonization by the acid- producing bacilli normally found in the vagina. The polyethylene glycol–based vaginal suppositories are water miscible and are generally suffi ciently fi rm for the patient to handle and insert without great diffi culty. However, to make the task easier, many manufacturers provide plastic insertion devices that are used to hold the suppository or tablet for proper placement within the vagina.
Packaging and Storage
Glycerin suppositories and glycerinated gelatin suppositories are packaged in tightly closed glass containers to prevent a change in moisture content. Suppositories prepared from a cocoa butter base are usually individually wrapped or otherwise separated in compartmented boxes to prevent contact and adhesion. Suppositories containing light-sensitive drugs are individually wrapped in an opaque material such as a metallic foil. In fact, most commercial suppositories are individually wrapped in either foil or plastic. Some are packaged in a continuous strip, separated by tearing along perforations. Suppositories are also commonly packaged in slide boxes or in plastic boxes.
Because suppositories are adversely affected by heat, it is necessary to maintain them in a cool place. Cocoa butter suppositories must be stored below 30°C (86°F), and preferably in a refrigerator (2°C to 8°C, or 36°F to 46°F). Glycerinated gelatin suppositories can be stored at controlled room temperature (20°C to 25°C, or 68°F to 77°F).
Suppositories made from a base of polyethylene glycol may be stored at usual room temperatures. Suppositories stored in high humidity may absorb moisture and tend to become spongy, whereas suppositories stored in places of extreme dryness may lose moisture and become brittle.
FINISHED PRODUCT
Manufacture:QUALITY CONTROL
Control Parameters of Suppositories:
FORMULATION OF VAGINAL DOSAGE FORMS
SELECTING A COMPOUNDING METHOD
By Hand Rolling
With ready availability of suppository molds of accommodating shapes and sizes, there is little requirement for today’s pharmacist to shape suppositories by hand. Hand rolling and shaping is a historic part of the art of the pharmacist.
By Fusion Molding
This step involves first melting the suppository base, and then dispersing or dissolving the drug in the melted base. The mixture is removed from the heat and poured into a suppository mold. When the mixture has congealed, the suppositories are removed from the mold. The fusion method can be used with all types of suppositories and must be used with most of them.
Suppositories are generally made from solid ingredients and drugs which are measured by weight. When they are mixed, melted, and poured into suppository mold cavities, they occupy a volume – the volume of the mold cavity. Since the components are measured by weight but compounded by volume, density calculations and mold calibrations are required to provide accurate doses.
When a drug is placed in a suppository base, it will displace an amount of base as a function of its density. If the drug has the same density as the base, it will displace an equivalent weight of the base. If the density of the drug is greater than that of the base, it will displace a proportionally smaller weight of the base. Density factors for common drugs in cocoa butter are available in standard reference texts. The density factor is used to determine how much of a base will be displaced by a drug.
By Compression
Suppositories may be prepared by forcing the mixed mass of the base and the medicaments into special molds using suppository-making machines. In preparation for compression into the molds, the base and the other formulative ingredients are combined by thorough mixing, the friction of the process softening the base into a pastelike consistency. On a small scale, a mortar and pestle may be used. Heating the mortar in warm water (then drying it) greatly facilitates the softening of the base and the mixing. On a large scale, a similar process may be used, employing mechanical kneading mixers and a warm mixing vessel.
Compression is especially suited for making suppositories that contain heat-labile medicinal substances or a great deal of substances that are insoluble in the base. In contrast to the molding method, compression permits no likelihood of insoluble matter settling during manufacture. The disadvantage to compression is that the special suppository machine is required and there is some limitation as to the shapes of suppositories that can be made.
In preparing suppositories with the compression machine, the suppository mass is placed in a cylinder; the cylinder is closed; pressure is applied from one end, mechanically or by turning a wheel; and the mass is forced out of the other end into the mold or die. When the die is filled with the mass, a movable end plate at the back of the die is removed, and when additional pressure is applied to the mass in the cylinder, the formed suppositories are ejected. The end plate is returned and the process is repeated until all of the mass has been used. Various sizes and shapes of dies are available. It is possible to prepare suppositories of uniform circumference by extrusion through a perforated plate and by cutting the extruded mass to the desired length.
By Molding
The steps in molding include (a) melting the base, (b) incorporating any required medicaments, (c) pouring the melt into molds, (d) allowing the melt to cool and congeal into suppositories, and (e) removing the formed suppositories from the mold. Cocoa butter, glycerinated gelatin, polyethylene glycol, and most other bases are suitable for preparation by molding
Sign up to our newsletter and receive the latest news and updates about our products!
