

Discovery to Delivery
› News ›Application and Technology News

A Cell Processing Isolator (CPI) is an integrated system that combines several types of equipment into one isolated solution. General bioprocessing equipment such as CO2 incubator and centrifuge can be integrated in the isolator.

The implementation of continuous manufacturing guarantees greater industrial efficiency and ultimately, the promotion of the nation’s overall health through the provision of affordable high quality medications.

Proper use of laboratory equipment is necessary to work safely with hazardous chemicals and processes. The maintenance and regular inspection of all laboratory apparatus are also vital, to prevent accidents linked with improper use of such equipment. Finding the right solution for the handling of apparatus and processes is currently needed, especially in start-up plants.

Esco Pharma introduces the newest member to its isolation technologies, the Isoclean® Healthcare Platform Isolator - Inflatable Seal Model (HPI-G3-IS). Isoclean® HPI is offered as an optimized solution for aseptic and potent compounding, sterility testing, aseptic filtration, cell and gene therapy, cosmeceuticals, and biocontainment, among others.

Esco Pharma introduces the newest member to its isolation technologies, the Isoclean® Healthcare Platform Isolator - Inflatable Seal Model (HPI-G3-IS). Isoclean® HPI is offered as an optimized solution for aseptic and potent compounding, sterility testing, aseptic filtration, cell and gene therapy, cosmeceuticals, and biocontainment, among others.

The entire process of pharmaceutical manufacturing involves a whole list of meticulous guidelines, both local and international, to adhere to. Moreover, it needs to pass the different stages of production before it can reach market platforms, and only then can the industry be assured of their product’s quality.
There have been many great feats made by the pharmaceutical industry in the past few years, but there is one that bothers both the healthcare and manufacturing industries continuous soar, the possibility of health decline and a high risk of bioburden.
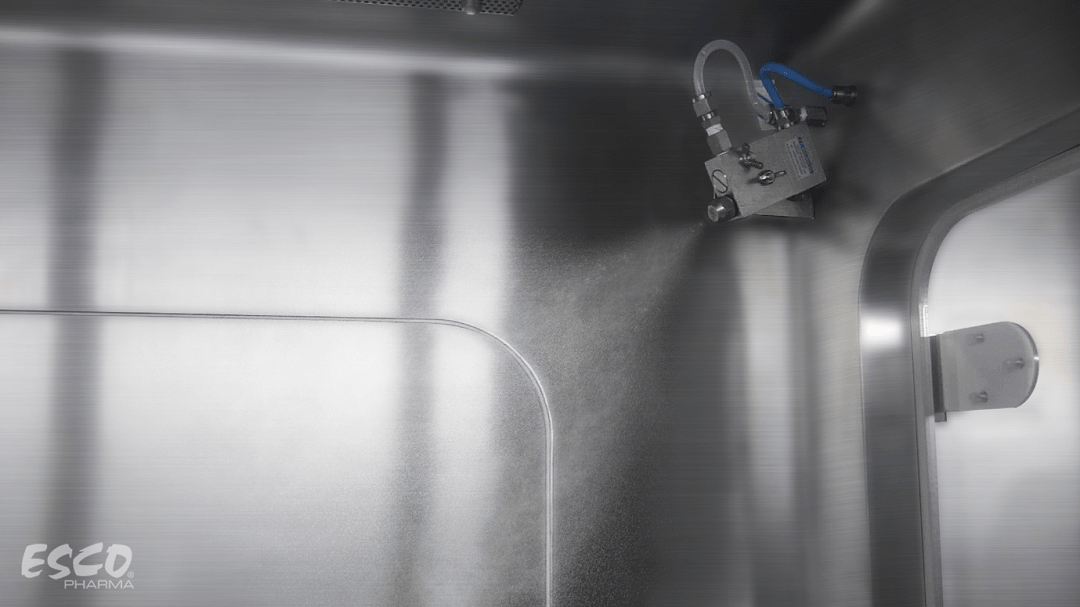
Contamination control is of extreme importance in biopharmaceutical industries, especially in maintaining product quality and sterility. As standards and guidelines evolved over time, biodecontamination became a part of the standard operating procedures (SOPs) for facilities who manufacture and handle high-quality products.
Sign up to our newsletter and receive the latest news and updates about our products!
